CCL5 is essential for axonogenesis and neuronal restoration after brain injury
- PMID: 39285280
- PMCID: PMC11406852
- DOI: 10.1186/s12929-024-01083-w
CCL5 is essential for axonogenesis and neuronal restoration after brain injury
Abstract
Background: Traumatic brain injury (TBI) causes axon tearing and synapse degradation, resulting in multiple neurological dysfunctions and exacerbation of early neurodegeneration; the repair of axonal and synaptic structures is critical for restoring neuronal function. C-C Motif Chemokine Ligand 5 (CCL5) shows many neuroprotective activities.
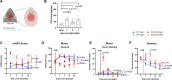
Method: A close-head weight-drop system was used to induce mild brain trauma in C57BL/6 (wild-type, WT) and CCL5 knockout (CCL5-KO) mice. The mNSS score, rotarod, beam walking, and sticker removal tests were used to assay neurological function after mTBI in different groups of mice. The restoration of motor and sensory functions was impaired in CCL5-KO mice after one month of injury, with swelling of axons and synapses from Golgi staining and reduced synaptic proteins-synaptophysin and PSD95. Administration of recombinant CCL5 (Pre-treatment: 300 pg/g once before injury; or post-treatment: 30 pg/g every 2 days, since 3 days after injury for 1 month) through intranasal delivery into mouse brain improved the motor and sensory neurological dysfunctions in CCL5-KO TBI mice.
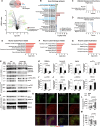
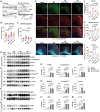
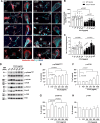
Results: Proteomic analysis using LC-MS/MS identified that the "Nervous system development and function"-related proteins, including axonogenesis, synaptogenesis, and myelination signaling pathways, were reduced in injured cortex of CCL5-KO mice; both pre-treatment and post-treatment with CCL5 augmented those pathways. Immunostaining and western blot analysis confirmed axonogenesis and synaptogenesis related Semaphorin, Ephrin, p70S6/mTOR signaling, and myelination-related Neuregulin/ErbB and FGF/FAK signaling pathways were up-regulated in the cortical tissue by CCL5 after brain injury. We also noticed cortex redevelopment after long-term administration of CCL5 after brain injury with increased Reelin positive Cajal-Rerzius Cells and CXCR4 expression. CCL5 enhanced the growth of cone filopodia in a primary neuron culture system; blocking CCL5's receptor CCR5 by Maraviroc reduced the intensity of filopodia in growth cone and also CCL5 mediated mTOR and Rho signalling activation. Inhibiting mTOR and Rho signaling abolished CCL5 induced growth cone formation.
Conclusions: CCL5 plays a critical role in starting the intrinsic neuronal regeneration system following TBI, which includes growth cone formation, axonogenesis and synaptogensis, remyelination, and the subsequent proper wiring of cortical circuits. Our study underscores the potential of CCL5 as a robust therapeutic stratagem in treating axonal injury and degeneration during the chronic phase after mild brain injury.
Keywords: Axon injury; Axonogenesis; CCL5; Myelination; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
CCL5 via GPX1 activation protects hippocampal memory function after mild traumatic brain injury.Redox Biol. 2021 Oct;46:102067. doi: 10.1016/j.redox.2021.102067. Epub 2021 Jul 17. Redox Biol. 2021. PMID: 34315111 Free PMC article.
-
Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice.Exp Neurol. 2006 Sep;201(1):253-65. doi: 10.1016/j.expneurol.2006.04.013. Epub 2006 Jun 30. Exp Neurol. 2006. PMID: 16814284
-
Genetic inactivation of SARM1 axon degeneration pathway improves outcome trajectory after experimental traumatic brain injury based on pathological, radiological, and functional measures.Acta Neuropathol Commun. 2021 May 17;9(1):89. doi: 10.1186/s40478-021-01193-8. Acta Neuropathol Commun. 2021. PMID: 34001261 Free PMC article.
-
Inhibition of EphA/Ephrin-A signaling using genetic and pharmacologic approaches improves recovery following traumatic brain injury in mice.Brain Inj. 2019;33(10):1385-1401. doi: 10.1080/02699052.2019.1641622. Epub 2019 Jul 18. Brain Inj. 2019. PMID: 31319723
-
Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes.Cell Death Dis. 2019 Feb 13;10(2):141. doi: 10.1038/s41419-019-1329-3. Cell Death Dis. 2019. PMID: 30760705 Free PMC article.
Cited by
-
Transcriptomic Redox Dysregulation in a Rat Model of Metabolic Syndrome-Associated Kidney Injury.Antioxidants (Basel). 2025 Jun 17;14(6):746. doi: 10.3390/antiox14060746. Antioxidants (Basel). 2025. PMID: 40563378 Free PMC article.
-
Olfactory mucosa-mesenchymal stem cells with overexpressed Nrf2 modulate angiogenesis and exert anti-inflammation effect in an in vitro traumatic brain injury model.Eur J Med Res. 2025 Feb 5;30(1):80. doi: 10.1186/s40001-025-02344-6. Eur J Med Res. 2025. PMID: 39910594 Free PMC article.
-
Mechanically-adaptive, resveratrol-eluting neural probes for improved intracortical recording performance and stability.Npj Flex Electron. 2025;9(1):64. doi: 10.1038/s41528-025-00440-5. Epub 2025 Jul 9. Npj Flex Electron. 2025. PMID: 40656548 Free PMC article.
-
Astrocyte-microglia crosstalk in subarachnoid hemorrhage: mechanisms and treatments.Front Immunol. 2025 Jun 30;16:1547858. doi: 10.3389/fimmu.2025.1547858. eCollection 2025. Front Immunol. 2025. PMID: 40661949 Free PMC article. Review.
References
-
- Ajoy R, Lo YC, Ho MH, Chen YY, Wang Y, Chen YH, Jing-Yuan C, Changou CA, Hsiung YC, Chen HM, Chang TH, Lee CY, Chiang YH, Chang WC, Hoffer B, Chou SY. CCL5 promotion of bioenergy metabolism is crucial for hippocampal synapse complex and memory formation. Mol Psychiatry. 2021;26(11):6451–68. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

