Prime-boost immunization with inactivated human adenovirus type 55 combined with an adjuvant enhances neutralizing antibody responses in mice
- PMID: 39285440
- PMCID: PMC11406814
- DOI: 10.1186/s12985-024-02491-y
Prime-boost immunization with inactivated human adenovirus type 55 combined with an adjuvant enhances neutralizing antibody responses in mice
Abstract
Background: Human adenovirus type 55 (hAd55) infection can lead to acute respiratory diseases that often present with severe symptoms. Despite its persistent prevalence in military camps and communities, there are no commercially available vaccines or vaccine candidates undergoing clinical evaluation; therefore, there is an urgent need to address this. In this study, we evaluated the immunogenicity of inactivated hAd55 isolates and investigated the effects of adjuvants and various immunization intervals.
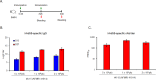
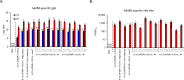
Methods and results: To select a vaccine candidate, four hAd55 strains (6-9, 6-15 (AFMRI 41014), 28-48 (AFMRI 41013), and 12-164 (AFMRI 41012)) were isolated from infected patients in military camps. Sequence analysis revealed no variation in the coding regions of structural proteins, including pentons, hexons, and fibers. Immunization with inactivated hAd55 isolates elicited robust hAd55-specific binding and neutralizing antibody responses in mice, with adjuvants, particularly alum hydroxide (AH), enhancing antibody titers. Co-immunization with AH also induced hAd14-specific neutralizing antibody responses but did not induce hAd11-specific neutralizing antibody responses. Notably, booster immunization administered at a four-week interval resulted in superior immune responses compared with shorter immunization intervals.
Conclusions: Prime-boost immunization with the inactivated hAd55 isolate and an AH adjuvant shows promise as a potential approach for preventing hAd55-induced respiratory disease. Further research is needed to evaluate the efficacy and safety of these vaccine candidates in preventing hAd55-associated respiratory illnesses.
Keywords: Acute respiratory disease; Human adenovirus type 55; Inactivated viral vaccine; Neutralizing antibody; Respiratory infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lynch JP 3rd, Kajon AE, Adenovirus. Epidemiology, global spread of Novel types, and Approach to Treatment. Semin Respir Crit Care Med. 2021;42(6):800–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

