The progress and future of the treatment of Candida albicans infections based on nanotechnology
- PMID: 39285480
- PMCID: PMC11406819
- DOI: 10.1186/s12951-024-02841-6
The progress and future of the treatment of Candida albicans infections based on nanotechnology
Abstract
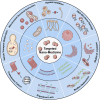
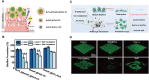
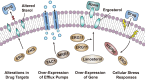
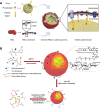
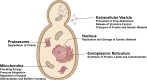
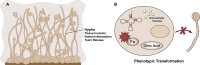
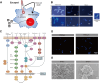
Systemic infection with Candida albicans poses a significant risk for people with weakened immune systems and carries a mortality rate of up to 60%. However, current therapeutic options have several limitations, including increasing drug tolerance, notable off-target effects, and severe adverse reactions. Over the past four decades, the progress in developing drugs to treat Candida albicans infections has been sluggish. This comprehensive review addresses the limitations of existing drugs and summarizes the efforts made toward redesigning and innovating existing or novel drugs through nanotechnology. The discussion explores the potential applications of nanomedicine in Candida albicans infections from four perspectives: nano-preparations for anti-biofilm therapy, innovative formulations of "old drugs" targeting the cell membrane and cell wall, reverse drug resistance therapy targeting subcellular organelles, and virulence deprivation therapy leveraging the unique polymorphism of Candida albicans. These therapeutic approaches are promising to address the above challenges and enhance the efficiency of drug development for Candida albicans infections. By harnessing nano-preparation technology to transform existing and preclinical drugs, novel therapeutic targets will be uncovered, providing effective solutions and broader horizons to improve patient survival rates.
Keywords: Candida albicans; Drug resistance; Hyphae; Nanomedicine; Subcellular organelles.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation.mBio. 2017 Dec 5;8(6):e01991-17. doi: 10.1128/mBio.01991-17. mBio. 2017. PMID: 29208749 Free PMC article.
-
Candida albicans: the current status regarding vaginal infections.Appl Microbiol Biotechnol. 2025 Apr 10;109(1):91. doi: 10.1007/s00253-025-13478-2. Appl Microbiol Biotechnol. 2025. PMID: 40210803 Free PMC article. Review.
-
Biofilm of Candida albicans: formation, regulation and resistance.J Appl Microbiol. 2021 Jul;131(1):11-22. doi: 10.1111/jam.14949. Epub 2020 Dec 9. J Appl Microbiol. 2021. PMID: 33249681 Review.
-
Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis.J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. J Mycol Med. 2020. PMID: 32008964
-
Magnesium deprivation affects cellular circuitry involved in drug resistance and virulence in Candida albicans.J Glob Antimicrob Resist. 2019 Jun;17:263-275. doi: 10.1016/j.jgar.2019.01.011. Epub 2019 Jan 16. J Glob Antimicrob Resist. 2019. PMID: 30659981
Cited by
-
ZnO Nanoparticle-Infused Vaterite Coatings: A Novel Approach for Antimicrobial Titanium Implant Surfaces.J Funct Biomater. 2025 Mar 19;16(3):108. doi: 10.3390/jfb16030108. J Funct Biomater. 2025. PMID: 40137388 Free PMC article.
References
-
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Reviews Disease Primers. 2018;4:1–20. - PubMed
-
- Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJ, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25:1200–12. - PubMed
-
- Hosseini-Moghaddam SM, Ouédraogo A, Naylor KL, Bota SE, Husain S, Nash DM, Paterson JM. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: a population‐based cohort study. Transpl Infect Disease. 2020;22:13250. - PubMed
-
- Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis. 2024;24:428–38. - PubMed
-
- Liu N, Zhou J, Jiang T, Tarsio M, Yu F, Zheng X, Qi W, Liu L, Tan J, Wei L, et al. A dual action small molecule enhances azoles and overcomes resistance through co-targeting Pdr5 and Vma1: Osimertinib targets Pdr5 and Vma1. Translational Research: J Lab Clin Med. 2022;247:39–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

