Association of anti-calcitonin gene-related peptide with other monoclonal antibodies for different diseases: A multicenter, prospective, cohort study
- PMID: 39285638
- PMCID: PMC11555159
- DOI: 10.1111/ene.16450
Association of anti-calcitonin gene-related peptide with other monoclonal antibodies for different diseases: A multicenter, prospective, cohort study
Abstract
Background and purpose: Although there is extensive evidence about the safety of monoclonal antibodies against calcitonin gene-related peptide (anti-CGRP mAbs) in combination with traditional drugs, scarce data are available on the safety of their combination with other mAbs. This study aimed to evaluate the 6-month effectiveness and tolerability of anti-CGRP mAbs in combination with other mAbs for different diseases.
Methods: Patients included in the Italian Headache Registry and treated concomitantly with an anti-CGRP mAb and another mAb were included. Effectiveness outcomes for migraine included reduction from baseline of monthly headache days (MHDs), Migraine Disability Assessment (MIDAS) score, Headache Impact Test-6 (HIT-6) scores, and Patients' Global Impression of Change (PGIC) scale. Adverse events (AEs) were recorded.
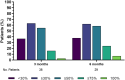
Results: Thirty-eight patients were included. In 27 patients (71.1%), the anti-CGRP mAb was added to a previously ongoing mAb. Nine patients (23.7%) discontinued one of the two mAbs before the end of treatment (seven discontinued the anti-CGRP mAb and two the other mAb). One patient discontinued for AEs. Anti-CGRP mAbs were discontinued due to ineffectiveness (n = 5, 55.5%) and one each (11.1%) for clinical remission and lost to follow-up. MHDs significantly decreased from baseline to 3 months (p < 0.0001) and 6 months (p < 0.001), as did the MIDAS and the HIT-6 scores at 3 and 6 months (p < 0.001). For anti-CGRP mAbs, 27.4% of patients reported PGIC ≥ 5 at 3 months and 48.3% at 6 months. Mild AEs associated with introduction of a second mAb were detected in six patients (15.8%).
Conclusions: In this real-world study, anti-CGRP mAbs showed safety and effectiveness when administered concomitantly with other mAbs.
Keywords: CGRP; migraine; monoclonal antibodies; pharmacokinetic; pharmacological interactions.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
L.F.I. has received fees and honoraria for advisory boards or speaker panels from Teva, Eli Lilly, Lundbeck, Pfizer, and AbbVie. G.Sap. has received fees and honoraria for advisory boards or speaker panels from Eli Lilly, Lundbeck, Pfizer, and Teva. G.V. has received personal fees from Lundbeck. M.A. has received personal fees from Eli Lilly, Lundbeck, Teva, Pfizer, and AbbVie/Allergan. P.C. has received research support, speaker honoraria, and support to attend national and international conferences from AbbVie, Bayer Schering, Bial, Biogen‐Dompè, Biogen‐Idec, Eisai, Genzyme, Lundbeck, Lusofarmaco, Merck‐Serono, Novartis, Prexton, Teva, UCB Pharma, and Zambon. R.O. reports personal fees and nonfinancial support from Allergan‐AbbVie, Eli Lilly, Novartis, Pfizer, and Teva. S.G. has received fees and honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Eli Lilly, Pfizer, Lundbeck, Angelini, and AbbVie. S.S. reports personal fees from Abbott, Allergan‐AbbVie, AstraZeneca, Boehringer, Eli Lilly, Lundbeck, Novartis, Novo Nordisk, Pfizer, and Teva and research grants from Novartis and Uriach. The other authors have no conflicting interests.
Figures


References
-
- Charles A, Pozo‐Rosich P. Targeting calcitonin gene‐related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765‐1774. - PubMed
-
- Silberstein SD, Dodick D, Freitag F, et al. Pharmacological approaches to managing migraine and associated comorbidities—clinical considerations for monotherapy versus polytherapy. Headache. 2007;47(4):585‐599. - PubMed
-
- Henricks LM, Schellens JH, Huitema AD, Beijnen JH. The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat Rev. 2015;41(10):859‐867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

