ANKRD1 aggravates renal ischaemia‒reperfusion injury via promoting TRIM25-mediated ubiquitination of ACSL3
- PMID: 39285846
- PMCID: PMC11406046
- DOI: 10.1002/ctm2.70024
ANKRD1 aggravates renal ischaemia‒reperfusion injury via promoting TRIM25-mediated ubiquitination of ACSL3
Abstract
Background: Renal ischaemia‒reperfusion injury (IRI) is the primary cause of acute kidney injury (AKI). To date, effective therapies for delaying renal IRI and postponing patient survival remain absent. Ankyrin repeat domain 1 (ANKRD1) has been implicated in some pathophysiologic processes, but its role in renal IRI has not been explored.
Methods: The mouse model of IRI-AKI and in vitro model were utilised to investigate the role of ANKRD1. Immunoprecipitation-mass spectrometry was performed to identify potential ANKRD1-interacting proteins. Protein‒protein interactions and protein ubiquitination were examined using immunoprecipitation and proximity ligation assay and immunoblotting, respectively. Cell viability, damage and lipid peroxidation were evaluated using biochemical and cellular techniques.
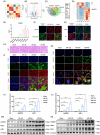
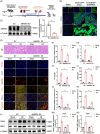
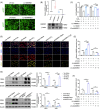
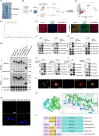
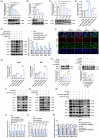
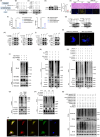
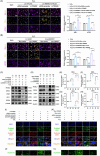
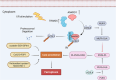
Results: First, we unveiled that ANKRD1 were significantly elevated in renal IRI models. Global knockdown of ANKRD1 in all cell types of mouse kidney by recombinant adeno-associated virus (rAAV9)-mitigated ischaemia/reperfusion-induced renal damage and failure. Silencing ANKRD1 enhanced cell viability and alleviated cell damage in human renal proximal tubule cells exposed to hypoxia reoxygenation or hydrogen peroxide, while ANKRD1 overexpression had the opposite effect. Second, we discovered that ANKRD1's detrimental function during renal IRI involves promoting lipid peroxidation and ferroptosis by directly binding to and decreasing levels of acyl-coenzyme A synthetase long-chain family member 3 (ACSL3), a key protein in lipid metabolism. Furthermore, attenuating ACSL3 in vivo through pharmaceutical approach and in vitro via RNA interference mitigated the anti-ferroptotic effect of ANKRD1 knockdown. Finally, we showed ANKRD1 facilitated post-translational degradation of ACSL3 by modulating E3 ligase tripartite motif containing 25 (TRIM25) to catalyse K63-linked ubiquitination of ACSL3, thereby amplifying lipid peroxidation and ferroptosis, exacerbating renal injury.
Conclusions: Our study revealed a previously unknown function of ANKRD1 in renal IRI. By driving ACSL3 ubiquitination and degradation, ANKRD1 aggravates ferroptosis and ultimately exacerbates IRI-AKI, underlining ANKRD1's potential as a therapeutic target for kidney IRI.
Key points/highlights: Ankyrin repeat domain 1 (ANKRD1) is rapidly activated in renal ischaemia‒reperfusion injury (IRI) models in vivo and in vitro. ANKRD1 knockdown mitigates kidney damage and preserves renal function. Ferroptosis contributes to the deteriorating function of ANKRD1 in renal IRI. ANKRD1 promotes acyl-coenzyme A synthetase long-chain family member 3 (ACSL3) degradation via the ubiquitin‒proteasome pathway. The E3 ligase tripartite motif containing 25 (TRIM25) is responsible for ANKRD1-mediated ubiquitination of ACSL3.
Keywords: ACSL3; ANKRD1; TRIM25; ferroptosis; renal ischaemic‒reperfusion injury; ubiquitination.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures


References
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199‐2210. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
