ECM-mimicking composite hydrogel for accelerated vascularized bone regeneration
- PMID: 39285909
- PMCID: PMC11404060
- DOI: 10.1016/j.bioactmat.2024.08.035
ECM-mimicking composite hydrogel for accelerated vascularized bone regeneration
Abstract
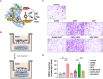
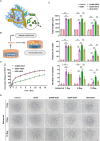
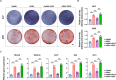
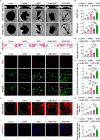
Bioactive hydrogel materials have great potential for applications in bone tissue engineering. However, fabrication of functional hydrogels that mimic the natural bone extracellular matrix (ECM) remains a challenge, because they need to provide mechanical support and embody physiological cues for angiogenesis and osteogenesis. Inspired by the features of ECM, we constructed a dual-component composite hydrogel comprising interpenetrating polymer networks of gelatin methacryloyl (GelMA) and deoxyribonucleic acid (DNA). Within the composite hydrogel, the GelMA network serves as the backbone for mechanical and biological stability, whereas the DNA network realizes dynamic capabilities (e.g., stress relaxation), thereby promoting cell proliferation and osteogenic differentiation. Furthermore, functional aptamers (Apt19S and AptV) are readily attached to the DNA network to recruit bone marrow mesenchymal stem cells (BMSCs) and achieve sustained release of loaded vascular endothelial growth factor towards angiogenesis. Our results showed that the composite hydrogel could facilitate the adhesion of BMSCs, promote osteogenic differentiation by activating focal adhesion kinase (FAK)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/β-Catenin signaling pathway, and eventually enhance vascularized bone regeneration. This study shows that the multifunctional composite hydrogel of GelMA and DNA can successfully simulate the biological functions of natural bone ECM and has great potential for repairing bone defects.
Keywords: Composite hydrogel; DNA hydrogel; Osteogenesis; Stress relaxation; Vascularization.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects.Mater Today Bio. 2022 Aug 8;16:100382. doi: 10.1016/j.mtbio.2022.100382. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36033373 Free PMC article.
-
[Study on the gelatin methacryloyl composite scaffold with exogenous transforming growth factor β 1 to promote the repair of skull defects].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 Jul 15;35(7):904-912. doi: 10.7507/1002-1892.202102008. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021. PMID: 34308601 Free PMC article. Chinese.
-
Amyloid Fibril and Clay Nanosheet Dual-Nanoengineered DNA Dynamic Hydrogel for Vascularized Bone Regeneration.ACS Nano. 2023 Sep 12;17(17):17131-17147. doi: 10.1021/acsnano.3c04816. Epub 2023 Aug 16. ACS Nano. 2023. PMID: 37585498
-
GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances.Biomater Res. 2023 Sep 15;27(1):86. doi: 10.1186/s40824-023-00422-6. Biomater Res. 2023. PMID: 37715230 Free PMC article. Review.
-
Advanced Hybrid Strategies of GelMA Composite Hydrogels in Bone Defect Repair.Polymers (Basel). 2024 Oct 29;16(21):3039. doi: 10.3390/polym16213039. Polymers (Basel). 2024. PMID: 39518248 Free PMC article. Review.
Cited by
-
Bionic Nanostructures Create Mechanical Signals to Mediate the Composite Structural Bone Regeneration Through Multi-System Regulation.Adv Sci (Weinh). 2025 Aug;12(31):e02299. doi: 10.1002/advs.202502299. Epub 2025 Jun 4. Adv Sci (Weinh). 2025. PMID: 40464259 Free PMC article. Review.
-
Sequential construction of vascularized and mineralized bone organoids using engineered ECM-DNA-CPO-based bionic matrix for efficient bone regeneration.Bioact Mater. 2025 Mar 14;49:362-377. doi: 10.1016/j.bioactmat.2025.02.033. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40144795 Free PMC article.
-
ECM remodeling by PDGFRβ+ dental pulp stem cells drives angiogenesis and pulp regeneration via integrin signaling.Stem Cell Res Ther. 2025 Jun 3;16(1):283. doi: 10.1186/s13287-025-04382-7. Stem Cell Res Ther. 2025. PMID: 40462244 Free PMC article.
-
Cyclodextrin-Based Supramolecular Hydrogels in Tissue Engineering and Regenerative Medicine.Molecules. 2025 Jul 31;30(15):3225. doi: 10.3390/molecules30153225. Molecules. 2025. PMID: 40807400 Free PMC article. Review.
-
Innovative Biocompatible Blend Scaffold of Poly(hydroxybutyrate-co-hydroxyvalerate) and Poly(ε-caprolactone) for Bone Tissue Engineering: In Vitro and In Vivo Evaluation.Polymers (Basel). 2024 Oct 30;16(21):3054. doi: 10.3390/polym16213054. Polymers (Basel). 2024. PMID: 39518269 Free PMC article.
References
-
- Kumar A., Rao K.M., Han S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017;317:119–131. doi: 10.1016/j.cej.2017.02.065. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

