Utility of Cerebrospinal Fluid Unstimulated Interferon-Gamma (IRISA-TB) as a Same-Day Test for Tuberculous Meningitis in a Tuberculosis-Endemic, Resource-Poor Setting
- PMID: 39286031
- PMCID: PMC11403475
- DOI: 10.1093/ofid/ofae496
Utility of Cerebrospinal Fluid Unstimulated Interferon-Gamma (IRISA-TB) as a Same-Day Test for Tuberculous Meningitis in a Tuberculosis-Endemic, Resource-Poor Setting
Erratum in
-
Correction to: Utility of Cerebrospinal Fluid Unstimulated Interferon-Gamma (IRISA-TB) as a Same-Day Test for Tuberculous Meningitis in a Tuberculosis-Endemic, Resource-Poor Setting.Open Forum Infect Dis. 2025 Mar 19;12(3):ofaf155. doi: 10.1093/ofid/ofaf155. eCollection 2025 Mar. Open Forum Infect Dis. 2025. PMID: 40110420 Free PMC article.
-
Correction to: Immunogenicity and Safety of the Higher-Valent Pneumococcal Conjugate Vaccine vs the 13-Valent Pneumococcal Conjugate Vaccine in Older Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Open Forum Infect Dis. 2025 Mar 20;12(3):ofaf169. doi: 10.1093/ofid/ofaf169. eCollection 2025 Mar. Open Forum Infect Dis. 2025. PMID: 40114974 Free PMC article.
Abstract
Background: Tuberculous meningitis (TBM) mortality is high and current diagnostics perform suboptimally. We evaluated the diagnostic performance of a DNA-based assay (GeneXpert Ultra) against a new same-day immunodiagnostic assay that detects unstimulated interferon-gamma (IRISA-TB).
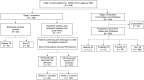
Methods: In a stage 1 evaluation, IRISA-TB was evaluated in biobanked samples from Zambia (n = 82; tuberculosis [TB] and non-TBM), and specificity in a South African biobank (n = 291; non-TBM only). Given encouraging results, a stage 2 evaluation was performed in suspected TBM patients from Zimbabwe and Malawi (n = 668). Patients were classified as having definite, probable or possible TBM, or non-TBM based on their microbiological results, cerebrospinal fluid (CSF) chemistry, and whether they received treatment.
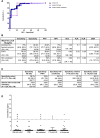
Results: In the stage 1 evaluation, sensitivity and specificity of IRISA-TB were 75% and 87% in the Zambian samples, and specificity was 100% in the South African samples. In the stage 2 validation, IRISA-TB sensitivity (95% confidence interval [CI]) was significantly higher than Xpert Ultra (76.2% [55.0%-89.4%] vs 25% [8.9%-53.3%]; P = .0048) when trace readouts were considered negative. Specificity (95% CI) was similar for both assays (91.4% [88.8%-93.4%] vs 86.9% [83.4%-89.8%]). When the Xpert Ultra polymerase chain reaction product was verified by sequencing, the positive predictive value of trace readouts in CSF was 27.8%. Sensitivity of IRISA-TB was higher in human immunodeficiency virus (HIV)-infected versus uninfected participants (85.8% vs 66.7%).
Conclusions: As a same-day rule-in test, IRISA-TB had significantly better sensitivity than Xpert Ultra in a TB/HIV-endemic setting. An immunodiagnostic approach to TBM is promising, and further studies are warranted.
Keywords: GeneXpert Ultra; accuracy; diagnosis; interferon gamma; tuberculous meningitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures


References
-
- World Health Organization (WHO) . Global tuberculosis report 2023. Geneva, Switzerland: WHO; 2023.
LinkOut - more resources
Full Text Sources
Research Materials

