Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake
- PMID: 39286244
- PMCID: PMC11403286
- DOI: 10.3389/fimmu.2024.1419527
Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake
Abstract
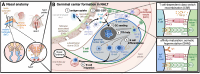
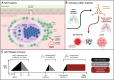
Mucosal barrier tissues and their mucosal associated lymphoid tissues (MALT) are attractive targets for vaccines and immunotherapies due to their roles in both priming and regulating adaptive immune responses. The upper and lower respiratory mucosae, in particular, possess unique properties: a vast surface area responsible for frontline protection against inhaled pathogens but also simultaneous tight regulation of homeostasis against a continuous backdrop of non-pathogenic antigen exposure. Within the upper and lower respiratory tract, the nasal and bronchial associated lymphoid tissues (NALT and BALT, respectively) are key sites where antigen-specific immune responses are orchestrated against inhaled antigens, serving as critical training grounds for adaptive immunity. Many infectious diseases are transmitted via respiratory mucosal sites, highlighting the need for vaccines that can activate resident frontline immune protection in these tissues to block infection. While traditional parenteral vaccines that are injected tend to elicit weak immunity in mucosal tissues, mucosal vaccines (i.e., that are administered intranasally) are capable of eliciting both systemic and mucosal immunity in tandem by initiating immune responses in the MALT. In contrast, administering antigen to mucosal tissues in the absence of adjuvant or costimulatory signals can instead induce antigen-specific tolerance by exploiting regulatory mechanisms inherent to MALT, holding potential for mucosal immunotherapies to treat autoimmunity. Yet despite being well motivated by mucosal biology, development of both mucosal subunit vaccines and immunotherapies has historically been plagued by poor drug delivery across mucosal barriers, resulting in weak efficacy, short-lived responses, and to-date a lack of clinical translation. Development of engineering strategies that can overcome barriers to mucosal delivery are thus critical for translation of mucosal subunit vaccines and immunotherapies. This review covers engineering strategies to enhance mucosal uptake via active targeting and passive transport mechanisms, with a parallel focus on mechanisms of immune activation and regulation in the respiratory mucosa. By combining engineering strategies for enhanced mucosal delivery with a better understanding of immune mechanisms in the NALT and BALT, we hope to illustrate the potential of these mucosal sites as targets for immunomodulation.
Keywords: adaptive immunity; antigen specific immunotherapy (ASIT); bronchus associated lymphoid tissue (BALT); drug delivery; germinal center (GC); mucosal vaccine; nasal associated lymphoid tissue (NALT); secretory IgA (SIgA).
Copyright © 2024 Seefeld, Templeton, Lehtinen, Sinclair, Yadav and Hartwell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Hartwell BL, Melo MB, Xiao P, Lemnios AA, Li N, Chang JYH, et al. . Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity. Sci Transl Med. (2022) 14:eabn1413–eabn1413. doi: 10.1126/scitranslmed.abn1413 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

