The prognostic utility of 18F-FDG PET parameters in lymphoma patients under CAR-T-cell therapy: a systematic review and meta-analysis
- PMID: 39286245
- PMCID: PMC11402741
- DOI: 10.3389/fimmu.2024.1424269
The prognostic utility of 18F-FDG PET parameters in lymphoma patients under CAR-T-cell therapy: a systematic review and meta-analysis
Abstract
Background: Chimeric antigen receptor (CAR) T-cell therapy has attracted considerable attention since its recent endorsement by the Food and Drug Administration, as it has emerged as a promising immunotherapeutic modality within the landscape of oncology. This study explores the prognostic utility of [18F]Fluorodeoxyglucose positron emission tomography ([18F]FDG PET) in lymphoma patients undergoing CAR T-cell therapy. Through meta-analysis, pooled hazard ratio (HR) values were calculated for specific PET metrics in this context.
Methods: PubMed, Scopus, and Ovid databases were explored to search for relevant topics. Dataset retrieval from inception until March 12, 2024, was carried out. The primary endpoints were impact of specific PET metrics on overall survival (OS) and progression-free survival (PFS) before and after treatment. Data from the studies were extracted for a meta-analysis using Stata 17.0.
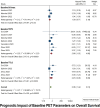
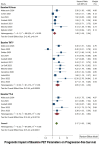
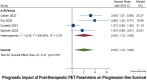
Results: Out of 27 studies identified for systematic review, 15 met the criteria for meta-analysis. Baseline OS analysis showed that total metabolic tumor volume (TMTV) had the highest HR of 2.66 (95% CI: 1.52-4.66), followed by Total-body total lesion glycolysis (TTLG) at 2.45 (95% CI: 0.98-6.08), and maximum standardized uptake values (SUVmax) at 1.30 (95% CI: 0.77-2.19). TMTV and TTLG were statistically significant (p < 0.0001), whereas SUVmax was not (p = 0.33). For PFS, TMTV again showed the highest HR at 2.65 (95% CI: 1.63-4.30), with TTLG at 2.35 (95% CI: 1.40-3.93), and SUVmax at 1.48 (95% CI: 1.08-2.04), all statistically significant (p ≤ 0.01). The ΔSUVmax was a significant predictor for PFS with an HR of 2.05 (95% CI: 1.13-3.69, p = 0.015).
Conclusion: [18F]FDG PET parameters are valuable prognostic tools for predicting outcome of lymphoma patients undergoing CAR T-cell therapy.
Keywords: CAR T cell therapy; CAR T-cell; FDG; chimeric antigen receptor; immunotherapy; meta-analysis; molecular imaging; systematic review.
Copyright © 2024 Al-Ibraheem, Abdlkadir, Al-Adhami, Sathekge, Bom, Ma’koseh, Mansour, Abdel-Razeq, Al-Rabi, Estrada-Lobato, Al-Hussaini, Matalka, Abdel Rahman and Fanti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

