Immunogenicity of a third dose with mRNA-vaccines in the ChAdOx1-S/BNT162b2 vaccination regimen against SARS-CoV-2 variants
- PMID: 39286494
- PMCID: PMC11404211
- DOI: 10.1016/j.isci.2024.110728
Immunogenicity of a third dose with mRNA-vaccines in the ChAdOx1-S/BNT162b2 vaccination regimen against SARS-CoV-2 variants
Abstract
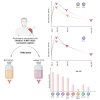
CombiVacS study has demonstrated a strong immune response of the heterologous ChAdOx1-S/BNT162b2 vaccine combination. The primary outcomes of the study were to assess the humoral immune response against SARS-CoV-2, 28 days after a third dose of a mRNA vaccine, in subjects that received a previous prime-boost scheme with ChAdOx1-S/BNT162b2. Secondary outcomes extended the study to 3 and 6 months. The third vaccine dose of mRNA-1273 in naive participants previously vaccinated with ChAdOx1-S/BNT162b2 regimen reached higher neutralizing antibodies titers against the variants of concern Delta and BA.1 lineage of Omicron compared with those receiving a third dose of BNT162b2 at day 28. These differences between BNT162b2 and mRNA-1273 arms were observed against the ancestral variant G614 at day 90. Suboptimal neutralizing response was observed against BQ.1.1, XBB.1.5/XBB.1.9, and JN.1 in a relevant proportion of individuals 180 days after the third dose, even after asymptomatic Omicron breakthrough infections. EudraCT (2021-001978-37); ClinicalTrials.gov (NCT04860739).
Keywords: Immune response; Immunology.
© 2024 The Authors.
Conflict of interest statement
J.A. has received fees for educational programs from Gilead, MSD, GSK and Janssen outside of the submitted work. M.C.-A. has participated in advisory boards and has received research funding from GSK, Sanofi Pasteur, Pfizer, Novavax, and Janssen. C.B.-I. is the deputy general manager of the Instituto de Salud Carlos III. J.R.A. has received fees from Janssen, outside of the submitted work. A.M.B. is principal investigator of clinical trials sponsored by GlaxoSmithKline, Daiichi-Sankyo, Janssen, and Farmalider, outside of the submitted work.
Figures



References
-
- Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., Lidegaard Ø., Tapia G., Gulseth H.L., Ruiz P.L.D., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1114. - DOI - PMC - PubMed
-
- Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., Campins M., Portolés A., González-Pérez M., García Morales M.T. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2022;398:121–130. - PMC - PubMed
-
- García-Pérez J., González-Pérez M., Castillo de la Osa M., Borobia A.M., Castaño L., Bertrán M.J., Campins M., Portolés A., Lora D., Bermejo M., et al. Immunogenic dynamics and SARS-CoV-2 variant neutralisation of the heterologous ChAdOx1-S/BNT162b2 vaccination: Secondary analysis of the randomised CombiVacS study. EClinicalMedicine. 2022;50 doi: 10.1016/j.eclinm.2022.101529. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

