Feasibility and barriers to rapid establishment of patient-derived primary osteosarcoma cell lines in clinical management
- PMID: 39286504
- PMCID: PMC11403063
- DOI: 10.1016/j.isci.2024.110251
Feasibility and barriers to rapid establishment of patient-derived primary osteosarcoma cell lines in clinical management
Abstract
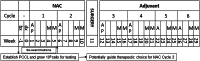
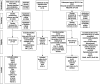
Osteosarcoma is a highly aggressive primary bone tumor that has seen little improvement in survival rates in the past three decades. Preclinical studies are conducted on a small pool of commercial cell lines which may not fully reflect the genetic heterogeneity of this complex cancer, potentially hindering translatability of in vitro results. Developing a single-site laboratory protocol to rapidly establish patient-derived primary cancer cell lines (PCCL) within a clinically actionable time frame of a few weeks will have significant scientific and clinical ramifications. These PCCL can widen the pool of available cell lines for study while patient-specific data could derive therapeutic correlation. This endeavor is exceedingly challenging considering the proposed time constraints. By proposing key definitions and a clear theoretical framework, this evaluation of osteosarcoma cell line establishment methodology over the past three decades assesses feasibility by identifying barriers and suggesting solutions, thereby facilitating systematic experimentation and optimization.
Keywords: Cancer; Technical aspects of cell biology.
© 2024 The Author(s).
Conflict of interest statement
This work was supported by Research Endowment Fund 2022 – St. Vincent’s Hospital Melbourne. The authors declare no competing interests.
Figures


References
-
- Rodrigues J., Sarmento B., Pereira C.L. Osteosarcoma tumor microenvironment: the key for the successful development of biologically relevant 3D in vitro models. In Vitro Models. 2022;1:5–27. doi: 10.1007/s44164-022-00008-x. - DOI
-
- Schott C.R., Koehne A.L., Sayles L.C., Young E.P., Luck C., Yu K., Lee A.G., Breese M.R., Leung S.G., Xu H., et al. Osteosarcoma PDX-Derived Cell Line Models for Preclinical Drug Evaluation Demonstrate Metastasis Inhibition by Dinaciclib through a Genome-Targeted Approach. Clin. Cancer Res. 2024;30:849–864. doi: 10.1158/1078-0432.ccr-23-0873. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

