Unbiased analysis of knee cartilage thickness change over three years after sprifermin vs. placebo treatment - A post-hoc analysis from the phase 2B FORWARD study
- PMID: 39286575
- PMCID: PMC11403365
- DOI: 10.1016/j.ocarto.2024.100513
Unbiased analysis of knee cartilage thickness change over three years after sprifermin vs. placebo treatment - A post-hoc analysis from the phase 2B FORWARD study
Abstract
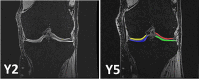
Objective: Post-treatment cartilage morphometry in the FORWARD study was performed without blinding to MRI acquisition order, involving potential reader bias. Here we obtained unbiased estimates of cartilage change post-treatment, reading year (Y)2 and Y5 MRIs with blinding to time point. We studied whether post-treatment cartilage thickness change differed between sprifermin- and placebo-treated knees.
Methods: FORWARD was a 5-year randomized control trial in 549 knee osteoarthritis patients. Here, Y2/Y5 images were analyzed with blinding to relative temporal order and treatment group. Cartilage change during Y2→Y5 was obtained in 337 participants: n = 57 treated with placebo intra-articular injections every 6 months (q6M); n = 69 with 30 μg sprifermin every 12 months (q12 M), n = 67 with 30 μg q6M, n = 73 with 100 μg q12 M, and n = 71 with 100 μg q6M between baseline (BL) and 18 M. Total femorotibial joint (TFTJ) cartilage thickness was the primary analytic focus.
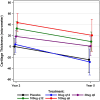
Results: TFTJ cartilage thickness change during Y2→Y5 was -26μm (SD64; 95%CI -32,-19) across the cohort; no statistically significant difference (p = 0.80) was observed between Sprifermin treated or placebo arms (one-way ANOVA). All groups lost cartilage, but the treatment-related difference in cartilage thickness in Sprifermin arms relative to placebo at Y2 was maintained until Y5. Annualized cartilage change in placebo participants was -8.2 μm (SD21; 95%CI -14,-2.5) during Y2→Y5 vs. -5.4 μm (SD27; 95%CI -13,1.8) during BL→Y2; no significant difference was identified (t-test).
Conclusion: FORWARD is the first study evaluating post-treatment benefits of a potential disease modifying osteoarthritis drug. Cartilage thickness gained with 100 μg sprifermin at Y2 is maintained to Y5 and thus appears viable and sustainable.This is a post-hoc analysis of the FORWARD trial: ClinicalTrials.gov Identifier: NCT01919164.
Keywords: Cartilage morphometry; Clinical trial; DMOAD (sprifermin); Magnetic resonance imaging; Post treatment efficacy.
© 2024 The Authors.
Conflict of interest statement
Felix Eckstein, Susanne Maschek, and Wolfgang Wirth are employees of Chondrometrics GmbH, a company that provides professional image analysis service to researchers in academia and to the pharmaceutical industry. They also are co-owners of Chondrometrics GmbH. They have received funding from multiple sources, including the pharmaceutical industry, Universities, and public national bodies (detailed list upon request). Felix Eckstein has provided consulting services to Merck KGA, Tissue Gene, Galapagos, Novartis, 4P Pharma/4 Moving, and TrialSpark/Formation Bio. Chris Ladel is a self-employed owner of CHL4special consulting. He has provided consulting services to Regenosine, Curnova, Charité hospital, TrialSpark/Formation Bio and ReumaNederland. He was formerly an employee of Merck KGaA, a company involved in the Sprifermin development program including the FORWARD trial. Asger Reinstrup-Bihlet is a shareholder and employee of NBCD A/S. He was formerly an employee of Nordik Bioscience, a company involved in the FORWARD trial. Chris Knight, Kenneth Somberg and Luping Zhao are shareholders and employees of Formation Bio, the company being the current owner of Sprifermin.
Figures
References
-
- Gigout A., Guehring H., Froemel D., Meurer A., Ladel C., Reker D., et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage. 2017;25(11):1858–1867. - PubMed
-
- Moore E.E., Bendele A.M., Thompson D.L., Littau A., Waggie K.S., Reardon B., et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:1063–4584. (Print)):623-631. - PubMed
-
- Eckstein F., Wluka A.E., Wirth W., Cicuttini F. 30 Years of MRI-based cartilage & bone morphometry in knee osteoarthritis: from correlation to clinical trials. Osteoarthritis Cartilage. 2024;32(4):439–451. - PubMed
-
- Dahlberg L.E., Aydemir A., Muurahainen N., Gühring H., Fredberg Edebo H., Krarup-Jensen N., et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin. Exp. Rheumatol. 2016;34(3):445–450. - PubMed
-
- Lohmander L.S., Hellot S., Dreher D., Krantz E.F.W., Kruger D.S., Guermazi A., et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2013;66(7):1820–1831. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

