Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks
- PMID: 39286601
- PMCID: PMC11404059
- DOI: 10.1016/j.obpill.2024.100127
Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks
Abstract
Background: This review investigates the side effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) like liraglutide, semaglutide, and tirzepatide, medications known for their efficacy in promoting weight loss among individuals with obesity. The rationale is rooted in understanding the balance between their therapeutic benefits and associated risks.
Methods: This was a comprehensive clinical review, including systematic reviews, meta-analyses, randomized controlled trials (RCTs), and cohort studies. Data were extracted from databases such as PubMed, Scopus, Embase, MEDLINE, and Google Scholar, focusing on the tolerability, severity, and risks of these medications.
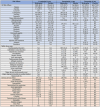
Results: GLP-1RAs demonstrated significant weight loss outcomes. In clinical trials, liraglutide showed a placebo-corrected weight loss of around 5 %, semaglutide 12 %, and tirzepatide 18 %. Common side effects were predominantly gastrointestinal, including nausea, diarrhea, constipation, and vomiting. Rare serious adverse events included gallbladder disorders and acute pancreatitis. In, addition, multiple studies identify new risks associated with GLP-1RAs including increased aspiration risk during anesthesia due to delayed gastric emptying and challenges with bowel preparation for colonoscopies.
Conclusion: While GLP-1RAs are effective in managing obesity, their use is associated with gastrointestinal side effects and rare but serious adverse events. The findings underscore the importance of individualized dosing and thorough patient assessment. Continuous research and vigilant monitoring are essential to optimize their safe use. Further studies are needed to refine guidelines, particularly regarding new concerns such as delayed gastric emptying and its implications for anesthesia.
Keywords: Cardiometabolic outcomes; GLP-1; Liraglutide; Semaglutide; Side effects; Tirzepatide.
© 2024 The Authors. Published by Elsevier Inc. on behalf of Obesity Medicine Association.
References
-
- Ward Z.J., et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. - PubMed
-
- Sørensen T.I.A., Martinez A.R., Jørgensen T.S.H. Epidemiology of obesity. Handb Exp Pharmacol. 2022;274:3–27. - PubMed
-
- Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–s185. - PubMed
-
- Waters H., Graf M. The health and economic costs of excess weight. Milken Institute; Santa Monica, California: 2018. America's obesity crisis.
Publication types
LinkOut - more resources
Full Text Sources