Vascular injury derived apoptotic exosome-like vesicles trigger autoimmunity
- PMID: 39286649
- PMCID: PMC11402544
- DOI: 10.1016/j.jtauto.2024.100250
Vascular injury derived apoptotic exosome-like vesicles trigger autoimmunity
Abstract
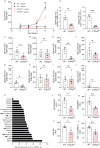
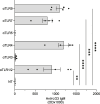
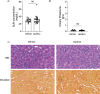
According to a central tenet of classical immune theory, a healthy immune system must avoid self-reactive lymphocyte clones but we now know that B cells repertoire exhibit some level of autoreactivity. These autoreactive B cells are thought to rely on self-ligands for their clonal selection and survival. Here, we confirm that healthy mice exhibit self-reactive B cell clones that can be stimulated in vitro by agonists of toll-like receptor (TLR) 1/2, TLR4, TLR7 and TLR9 to secrete anti-LG3/perlecan. LG3/perlecan is an antigen packaged in exosome-like structures released by apoptotic endothelial cells (ApoExos) upon vascular injury. We demonstrate that the injection of ApoExos in healthy animals activates the IL-23/IL-17 pro-inflammatory and autoimmune axis, and produces several autoantibodies, including anti-LG3 autoantibodies and hallmark autoantibodies found in systemic lupus erythematosus. We also identify γδT cells as key mediators of the maturation of ApoExos-induced autoantibodies in healthy mice. Altogether we show that ApoExos released by apoptotic endothelial cells display immune-mediating functions that can stimulate the B cells in the normal repertoire to produce autoantibodies. Our work also identifies TLR activation and γδT cells as important modulators of the humoral autoimmune response induced by ApoExos.
Keywords: Anti-LG3; ApoExos; Autoantibodies; Systemic lupus erythematosus (SLE); Toll-like receptors (TLR).
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources

