Curcumin's effect in advanced and metastatic breast cancer patients treated with first or second-line docetaxel: A randomized trial
- PMID: 39286738
- PMCID: PMC11403303
- DOI: 10.1002/hsr2.70052
Curcumin's effect in advanced and metastatic breast cancer patients treated with first or second-line docetaxel: A randomized trial
Abstract
Background: In this study, we investigated whether the association of curcumin and docetaxel among advanced and metastatic breast cancer patients in first or second-line treatment potentiated the objective response rate.
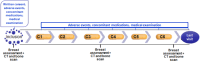
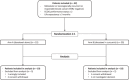
Patients/methods: A multicentre, randomized, open label, phase-II study was conducted and included 42 patients from July 2009 to January 2017. The primary endpoint was the objective response rate of the docetaxel-curcumin combination in comparison with docetaxel alone. The secondary endpoints were the assessment of clinical benefit, overall survival, time-to-progression, progression-free survival, compliance, and safety. An interim analysis was planned to evaluate safety and efficacy.
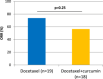
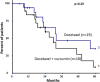
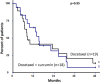
Results: In this interim analysis conducted on 37 patients (19 in the control group vs. 18 in the experimental group), no difference was observed for the objective response rate (p = 0.25, control 73.7% vs. experimental 55.6%). Concerning clinical benefit, overall survival and time-to-progression, we also failed to show any difference between the two arms. A slight tendency towards longer progression-free survival at 12 months after randomization was observed in the curcumin group (65.5% vs. 41.4%) but this difference did not reach significance (p = 0.14).
Conclusion: In this study, we showed for the first time that adding oral curcumin for advanced and metastatic breast cancer patients treated with first or second-line docetaxel was not efficacious, although safe. Consequently, this study was stopped for reasons of futility. Further studies with a larger number of patients, a different curcumin preparation, a longer treatment period and a pharmacokinetic evaluation of curcumin are needed to explore the real efficacy of this compound.
Keywords: advanced and metastatic breast cancer; chemotherapy; curcumin; docetaxel; phytotherapy.
© 2024 Centre Jean Perrin and The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Figures
References
LinkOut - more resources
Full Text Sources

