Lamellipodia-Mediated Osteoblast Haptotaxis Guided by Fibronectin Ligand Concentrations on a Multiplex Chip
- PMID: 39286887
- PMCID: PMC11618712
- DOI: 10.1002/smll.202401717
Lamellipodia-Mediated Osteoblast Haptotaxis Guided by Fibronectin Ligand Concentrations on a Multiplex Chip
Abstract
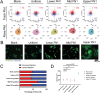
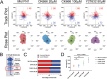
Skull morphogenesis is a complex, dynamic process involving two different germ layers and progressing to the coordinated, directional growth of individual bones. The mechanisms underlying directional growth toward the apex are not completely understood. Here, a microfluidic chip-based approach is utilized to test whether calvarial osteoblast progenitors undergo haptotaxis on a gradient of Fibronectin1 (FN1) via lamellipodia. Mimicking the embryonic cranial mesenchyme's FN1 pattern, FN1 gradients is established in the chip using computer modeling and fluorescent labeling. Primary mouse calvarial osteoblast progenitors are plated in the chip along an array of segmented gradients of adsorbed FN1. The study performs single-cell tracking and measures protrusive activity. Haptotaxis is observed at an intermediate FN1 concentration, with an average directional migration index (yFMI) of 0.07, showing a significant increase compared to the control average yFMI of -0.01. A significant increase in protrusive activity is observed during haptotaxis. Haptotaxis is an Arp2/3-dependent, lamellipodia-mediated process. Calvarial osteoblast progenitors treated with the Arp2/3 (Actin Related Protein 2/3 complex) inhibitor CK666 show significantly diminished haptotaxis, with an average yFMI of 0.01. Together, these results demonstrate haptotaxis on an FN1 gradient as a new mechanism in the apical expansion of calvarial osteoblast progenitors during development and shed light on the etiology of calvarial defects.
Keywords: fibronectin gradient; haptotaxis; lamellipodia; microfluidic; skull morphogenesis.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Lamellipodia are crucial for haptotactic sensing and response.J Cell Sci. 2016 Jun 15;129(12):2329-42. doi: 10.1242/jcs.184507. Epub 2016 May 12. J Cell Sci. 2016. PMID: 27173494 Free PMC article.
-
Apical expansion of calvarial osteoblasts and suture patency is dependent on fibronectin cues.Development. 2024 Apr 1;151(7):dev202371. doi: 10.1242/dev.202371. Epub 2024 Apr 11. Development. 2024. PMID: 38602508 Free PMC article.
-
Apical expansion of calvarial osteoblasts and suture patency is dependent on graded fibronectin cues.bioRxiv [Preprint]. 2023 Jan 16:2023.01.16.524278. doi: 10.1101/2023.01.16.524278. bioRxiv. 2023. Update in: Development. 2024 Apr 1;151(7):dev202371. doi: 10.1242/dev.202371. PMID: 36711975 Free PMC article. Updated. Preprint.
-
Steering cell migration: lamellipodium dynamics and the regulation of directional persistence.Nat Rev Mol Cell Biol. 2014 Sep;15(9):577-90. doi: 10.1038/nrm3861. Nat Rev Mol Cell Biol. 2014. PMID: 25145849 Review.
-
Membrane and actin dynamics interplay at lamellipodia leading edge.Curr Opin Cell Biol. 2013 Oct;25(5):565-73. doi: 10.1016/j.ceb.2013.04.001. Epub 2013 Apr 29. Curr Opin Cell Biol. 2013. PMID: 23639310 Review.
Cited by
-
Tunable methacrylated decellularized heart matrix: a versatile scaffold for cardiac tissue engineering.Front Bioeng Biotechnol. 2025 Jun 12;13:1579246. doi: 10.3389/fbioe.2025.1579246. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40574825 Free PMC article.
References
-
- Yoshida T., Vivatbutsiri P., Morriss‐Kay G., Saga Y., Iseki S., Mech. Dev. 2008, 125, 797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

