Worldwide CTEPH Registry: Long-Term Outcomes With Pulmonary Endarterectomy, Balloon Pulmonary Angioplasty, and Medical Therapy
- PMID: 39286890
- PMCID: PMC11562489
- DOI: 10.1161/CIRCULATIONAHA.124.068610
Worldwide CTEPH Registry: Long-Term Outcomes With Pulmonary Endarterectomy, Balloon Pulmonary Angioplasty, and Medical Therapy
Abstract
Background: The European Chronic Thromboembolic Pulmonary Hypertension (CTEPH) registry, conducted between 2007 and 2012, reported the major impact of pulmonary endarterectomy (PEA) on the long-term survival of patients with CTEPH. Since then, 2 additional treatments for inoperable CTEPH have become available: balloon pulmonary angioplasty (BPA), and an approved oral drug therapy with the guanylate cyclase stimulator riociguat. The current registry aimed to evaluate the effect of these new therapeutic approaches in a worldwide context.
Methods: Participation in this international global registry included 34 centers in 20 countries. Between February 2015 and September 2016, 1009 newly diagnosed, consecutive patients were included and followed until September 2019.
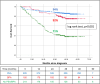
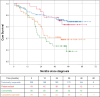
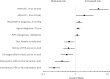
Results: Overall, 605 patients (60%) underwent PEA and 185 (18%) underwent BPA; 76% of the 219 remaining patients not receiving mechanical intervention (ie, neither PEA nor BPA) were treated with pulmonary hypertension drugs. Of patients undergoing PEA and BPA, 38% and 78% also received drugs for pulmonary hypertension, respectively. Median age at diagnosis was higher in the BPA and No PEA/BPA groups than in the PEA group: 66 and 69, respectively, versus 60 years. Pulmonary vascular resistance (PVR) was similar in all groups, with an average of 643 dynes.s.cm-5. During the observation period (>3 years; ≤5.6 years), death was reported in 7%, 11%, and 27% of patients treated by PEA and BPA, and those receiving no mechanical intervention (P<0.001). In Kaplan-Meier analysis, 3-year survival was 94%, 92%, and 71% in the 3 groups, respectively. PEA 3-year survival improved by 5% from that observed between 2007 and 2012. There was no survival difference in patients receiving vitamin K antagonists and non-vitamin K oral anticoagulants (P=0.756). In Cox regression, reduced mortality was associated with: PEA and BPA in the global cohort; history of venous thromboembolism and lower PVR in the PEA group; lower right atrial pressure in the BPA group; and use of pulmonary hypertension drugs, oxygen therapy, and lower right atrial pressure, as well as functional class in the group receiving no mechanical intervention.
Conclusions: This second international CTEPH registry reveals important improvement in patient survival since the introduction of BPA and an approved drug for pulmonary hypertension. The type of anticoagulation regimen did not influence survival.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02656238.
Keywords: angioplasty, balloon; endarterectomy; hypertension, pulmonary; registries; venous thromboembolism.
Conflict of interest statement
M.D. reports research grants from Janssen, speaker and consultant fees from Altavant, Acceleron, AOP, Bayer, Ferrer, Gossamer, INARI, Janssen, United Therapeutics, and MSD outside the submitted work, and all paid to her institution. She was holder of a Janssen chair for pulmonary hypertension at the KU Leuven. J.P.Z. reports research grants from MSD and consultant fees from Ferrer, Gossamer, Janssen outside submitted work. S.G. reports speaker fees or consultant honoraria from Actelion/Janssen, Bayer AG, MSD, and Pfizer. D.P.J. reports speaker fees and consultancy fees from Janssen outside this submitted work. D.G.K. reports speaker fees, consultancy fees, or funding to attend meetings from Acceleron, Altavant, Ferrer, Janssen, Gossamer, MSD and United Therapeutics and research support from Ferrer and Janssen, all outside of the submitted work. N.H.K. reports receiving speaker fees from Bayer and Janssen; consultancy fees from Bayer, Janssen, Merck, Polarean, Pulnovo, and United Therapeutics; and research support from Altavant and Gossamer Bio. All disclosed fees and support are outside the submitted work. M.M.M. reports consultancy fees and royalties from Wexler Surgical and consultancy fees from Actelion/Janssen and Johnson & Johnson. H.M. reports research grants from Nippon Shinyaku; speaker and consultant fees from Bayer, Janssen, and MSD; speaker fees from Kaneka Medix, Mochida, Nippon Shinyaku, and Nipro, all outside of the submitted work. J.S.O.A. reports personal fees from Bayer and MSD. R.S.K. reports having received speaker fees from AOP Health, Actelion/Janssen, and MSD, all outside of the submitted work. GS reports receiving advisory board and speaker fees from Acceleron, Bayer, Janssen, MSD, and Merck. C.B.W. reports speaker fees or consultant honoraria from Actelion/Janssen, AOP Orphan Pharmaceuticals AG, Bayer AG, BTG, MSD, OrphaCare, and Pfizer. E.M. reports receiving consultancy or speaker fees from Actelion/Janssen, Bayer, and MSD. I.M.L. has relationships with drug companies including AOP Health, Actelion/Janssen, MSD, United Therapeutics, Pulnovo, Medtronic, Neutrolis, and Sanofi; in addition to being investigator in trials involving these companies, relationships include consultancy services, research grants, and membership of scientific advisory boards. The other authors report no conflicts.
Figures

References
-
- Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, et al. . Chronic thromboembolic pulmonary hypertension (CTEPH). Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008 - PubMed
-
- Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, Bresser P, Torbicki A, Mellemkjaer S, Lewczuk J, et al. . Long-term outcome of patients with chronic thromboembolic pulmonary hypertension. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522 - PubMed
-
- Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, et al. ; CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657 - PubMed
-
- Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 - PubMed
-
- Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, Tatebe S, Miyamichi-Yamamoto S, Shimokawa H. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76:485–488. doi: 10.1253/circj.cj-11-1217 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

