DORA: 48-week weight and metabolic changes in Black women with HIV, in a phase IIIb switch study from dolutegravir- or efavirenz- to doravirine-based first-line antiretroviral therapy
- PMID: 39286902
- PMCID: PMC11725414
- DOI: 10.1111/hiv.13711
DORA: 48-week weight and metabolic changes in Black women with HIV, in a phase IIIb switch study from dolutegravir- or efavirenz- to doravirine-based first-line antiretroviral therapy
Abstract
Objectives: Treatment-related weight gain and metabolic complications with antiretroviral integrase-based regimens, especially among Black women, suggest the need for alternative options.
Methods: We conducted a 48-week, open-label, single-arm, single-centre, phase IIIb switch study to evaluate the tolerability, safety and efficacy of switching from stable efavirenz- or dolutegravir-based antiretroviral therapy to doravirine/lamivudine/tenofovir disoproxil fumarate in Black women.
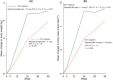
Results: The 101 participants enrolled (median age 35 years; interquartile range 31-40) were on efavirenz (n = 46; mean duration on therapy 1.7 years) or dolutegravir-based (n = 55; mean duration 1.5 years) antiretrovirals at screening. Retention at 48 weeks was 92/101 participants, and viral suppression was >90% throughout the study, with a single case of doravirine resistance (106 M, V108I and H221Y mutations). The mean weight percentage change at week 48 was 4.7% (95% confidence interval [CI] 3.0-6.5; p < 0.001), and the adjusted mean change was 2.7 kg (95% CI 1.50-3.98; p < 0.001); for efavirenz, the percentage change was 5.0% (95% CI 2.9-7.1; p < 0.001), and the adjusted weight gain was 3.5 kg (95% CI 1.93-5.13); for dolutegravir, the percentage change was 4.5% (95% CI 1.8-7.3; p < 0.001), and the adjusted weight gain was 2.1 kg (95% CI 0.26-3.90). Statistically significant decreases in lipid panel percent mean to week 48 included: total cholesterol -8.4% (95% CI -11.3 to -5.5; p < 0.001), triglycerides -10.4% (95% CI -16.4 to -4.4; p < 0.001) and high-density lipoprotein -14.8% (95% CI -18.5 to -11.2%; p < 0.001), with minor differences when disaggregating the mean percent change in lipids between previous efavirenz/dolutegravir regimens. Adverse events due to doravirine were few and mild.
Conclusions: Our findings suggest that a switch to doravirine from efavirenz or dolutegravir is safe and effective in Black women, with significant improvement in lipid profiles, but does not arrest progressive weight gain.
Keywords: antiretroviral; black; doravirine; female; metabolic.
© 2024 The Author(s). HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
AH has conducted statistical analysis for HIV‐related and tuberculosis‐related clinical trials for MetaVirology. MM joined ViiV Healthcare, as a full‐time employee as Head of Global Medical Directors in August 2020. WDFV's unit (Ezintsha) receives funding from the Bill and Melinda Gates Foundation, SA Medical Research Council, National Institutes for Health, Unitaid, Foundation for Innovative New Diagnostics (FIND), Merck and the Children's Investment Fund Foundation (CIFF); has previously received funding from USAID; and receives drug donations from ViiV Healthcare, Merck, J&J and Gilead Sciences for investigator‐led clinical studies. The unit conducts investigator‐led studies for which Merck, J&J and ViiV provide financial support and is conducting commercial drug studies for Merck. The unit performs evaluations of diagnostic devices for multiple biotech companies. Individually, he receives honoraria for educational talks and advisory board membership for Gilead, ViiV, Mylan/Viatris, Merck, Adcock‐Ingram, Aspen, Abbott, Roche, J&J, Sanofi and Virology Education.
Figures



References
-
- UNAIDS. FACT SHEET. 2023. [Internet]. [cited 2023 Jul 3]. Available from: chrome‐extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_...
-
- Voight E. Over 3million people on new HIV drug, but not all smooth sailing. Spotlight [Internet]. 2022. Available from: INSTIs initiation was associated with an early onset, excess incidence of cardiovascular disease in the first 2 years of%0Dexposure, after accounting for known cardiovascular disease risk factors.
-
- Clinton Health Access Initiative . 2023 HIV Market Report 2023 [Internet]. [cited 2024 Jan 3]. Available from: https://chai19.wpenginepowered.com/wp‐content/uploads/2023/11/2023‐HIV‐M....
-
- Neesgaard B, Greenberg L, Miró JM, et al. Associations between integrase strand‐transfer inhibitors and cardiovascular disease in people living with HIV: a multicentre prospective study from the RESPOND cohort consortium. Lancet HIV. 2022;9:e485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

