Control of Dynamic Chirality in Donor-Acceptor Fluorophores
- PMID: 39286924
- PMCID: PMC11720384
- DOI: 10.1002/anie.202414490
Control of Dynamic Chirality in Donor-Acceptor Fluorophores
Abstract
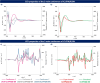
Recently, the control of dynamic chirality has emerged as a powerful strategy to design chiral functional materials. In this context, we describe herein a molecular design in which a tethered configurationally stable binaphthyl chiral unit efficiently controls the dynamic chirality of donor-acceptor fluorophores, involving diverse indolocarbazoles as electron donors and terephthalonitrile as an electron acceptor. The high conformational discrimination in such a molecular system suggested by density functional theory calculations is experimentally probed using electronic and vibrational circular dichroism and confirmed by the crystallization of these chiral molecules in gel and their single crystal X-ray diffraction analysis. This work also highlights the positive effect of the configurationally stable chiral unit on the magnitude of the dissymmetry factors of the active dynamically chiral fluorophores, both in ground and excited states, through chiral perturbation.
Keywords: Chiral materials; Circularly polarized luminescence; Dissymmetry factor; Electronic circular dichroism; Vibrational circular dichroism.
© 2024 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Crassous J., Fuchter M. J., Freedman D. E., Kotov N. A., Moon J., Beard M. C., Feldmann S., Nat. Rev. Mater. 2023, 8, 365–371.
-
- Brandt J. R., Salerno F., Fuchter M. J., Nat. Rev. Chem. 2017, 1, 1–12.
-
- Wu X., Yan X., Chen Y., Zhu W., Chou P.-T., Trends in Chemistry 2023, 5, 734–747.
-
- Frédéric L., Desmarchelier A., Favereau L., Pieters G., Adv. Funct. Mat. 2021, 31, 2010281.
-
- Wu X., Yan X., Chen Y., Zhu W., Chou P.-T., Trends in Chemistry 2023, 5, 734–747.
Grants and funding
LinkOut - more resources
Full Text Sources

