BCL2 expression is enriched in advanced prostate cancer with features of lineage plasticity
- PMID: 39286979
- PMCID: PMC11405043
- DOI: 10.1172/JCI179998
BCL2 expression is enriched in advanced prostate cancer with features of lineage plasticity
Abstract
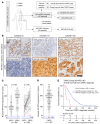
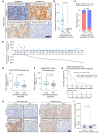
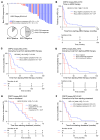
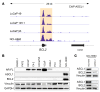
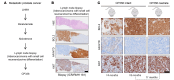
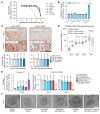
The widespread use of potent androgen receptor signaling inhibitors (ARSIs) has led to an increasing emergence of AR-independent castration-resistant prostate cancer (CRPC), typically driven by loss of AR expression, lineage plasticity, and transformation to prostate cancers (PCs) that exhibit phenotypes of neuroendocrine or basal-like cells. The anti-apoptotic protein BCL2 is upregulated in neuroendocrine cancers and may be a therapeutic target for this aggressive PC disease subset. There is an unmet clinical need, therefore, to clinically characterize BCL2 expression in metastatic CRPC (mCRPC), determine its association with AR expression, uncover its mechanisms of regulation, and evaluate BCL2 as a therapeutic target and/or biomarker with clinical utility. Here, using multiple PC biopsy cohorts and models, we demonstrate that BCL2 expression is enriched in AR-negative mCRPC, associating with shorter overall survival and resistance to ARSIs. Moreover, high BCL2 expression associates with lineage plasticity features and neuroendocrine marker positivity. We provide evidence that BCL2 expression is regulated by DNA methylation, associated with epithelial-mesenchymal transition, and increased by the neuronal transcription factor ASCL1. Finally, BCL2 inhibition had antitumor activity in some, but not all, BCL2-positive PC models, highlighting the need for combination strategies to enhance tumor cell apoptosis and enrich response.
Keywords: Apoptosis; Oncology; Prostate cancer.
Figures



References
-
- World Health Organization. Global Cancer Observatory. https://gco.iarc.fr/ Accessed January 13, 2022.
-
- Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–130. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

