Genetic requirements for uropathogenic E. coli proliferation in the bladder cell infection cycle
- PMID: 39287381
- PMCID: PMC11495030
- DOI: 10.1128/msystems.00387-24
Genetic requirements for uropathogenic E. coli proliferation in the bladder cell infection cycle
Abstract
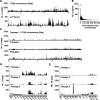
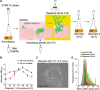
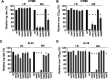
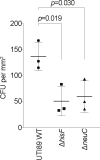
Uropathogenic Escherichia coli (UPEC) requires an adaptable physiology to survive the wide range of environments experienced in the host, including gut and urinary tract surfaces. To identify UPEC genes required during intracellular infection, we developed a transposon-directed insertion-site sequencing approach for cellular infection models and searched for genes in a library of ~20,000 UTI89 transposon-insertion mutants that are specifically required at the distinct stages of infection of cultured bladder epithelial cells. Some of the bacterial functional requirements apparent in host bladder cell growth overlapped with those for M9-glycerol, notably nutrient utilization, polysaccharide and macromolecule precursor biosynthesis, and cell envelope stress tolerance. Two genes implicated in the intracellular bladder cell infection stage were confirmed through independent gene deletion studies: neuC (sialic acid capsule biosynthesis) and hisF (histidine biosynthesis). Distinct sets of UPEC genes were also implicated in bacterial dispersal, where UPEC erupts from bladder cells in highly filamentous or motile forms upon exposure to human urine, and during recovery from infection in a rich medium. We confirm that the dedD gene linked to septal peptidoglycan remodeling is required during UPEC dispersal from human bladder cells and may help stabilize cell division or the cell wall during envelope stress created by host cells. Our findings support a view that the host intracellular environment and infection cycle are multi-nutrient limited and create stress that demands an array of biosynthetic, cell envelope integrity, and biofilm-related functions of UPEC.
Importance: Urinary tract infections (UTIs) are one of the most frequent infections worldwide. Uropathogenic Escherichia coli (UPEC), which accounts for ~80% of UTIs, must rapidly adapt to highly variable host environments, such as the gut, bladder sub-surface, and urine. In this study, we searched for UPEC genes required for bacterial growth and survival throughout the cellular infection cycle. Genes required for de novo synthesis of biomolecules and cell envelope integrity appeared to be important, and other genes were also implicated in bacterial dispersal and recovery from infection of cultured bladder cells. With further studies of individual gene function, their potential as therapeutic targets may be realized. This study expands knowledge of the UTI cycle and establishes an approach to genome-wide functional analyses of stage-resolved microbial infections.
Keywords: TraDIS; UPEC; UTI; cystitis; intracellular infection; stage-resolved model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
