AI for Multistructure Incidental Findings and Mortality Prediction at Chest CT in Lung Cancer Screening
- PMID: 39287522
- PMCID: PMC11427857
- DOI: 10.1148/radiol.240541
AI for Multistructure Incidental Findings and Mortality Prediction at Chest CT in Lung Cancer Screening
Abstract
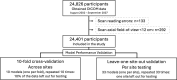
Background Incidental extrapulmonary findings are commonly detected on chest CT scans and can be clinically important. Purpose To integrate artificial intelligence (AI)-based segmentation for multiple structures, coronary artery calcium (CAC), and epicardial adipose tissue with automated feature extraction methods and machine learning to detect extrapulmonary abnormalities and predict all-cause mortality (ACM) in a large multicenter cohort. Materials and Methods In this post hoc analysis, baseline chest CT scans in patients enrolled in the National Lung Screening Trial (NLST) from August 2002 to September 2007 were included from 33 participating sites. Per scan, 32 structures were segmented with a multistructure model. For each structure, 15 clinically interpretable radiomic features were quantified. Four general codes describing abnormalities reported by NLST radiologists were applied to identify extrapulmonary significant incidental findings on the CT scans. Death at 2-year and 10-year follow-up and the presence of extrapulmonary significant incidental findings were predicted with ensemble AI models, and individualized structure risk scores were evaluated. Area under the receiver operating characteristic curve (AUC) analysis was used to evaluate the performance of the models for prediction of ACM and extrapulmonary significant incidental findings. The Pearson χ2 test and Kruskal-Wallis rank sum test were used for statistical analyses. Results A total of 24 401 participants (median age, 61 years [IQR, 57-65 years]; 14 468 male) were included. In 3880 of 24 401 participants (16%), 4283 extrapulmonary significant incidental findings were reported. During the 10-year follow-up, 3389 of 24 401 participants (14%) died. CAC had the highest feature importance for predicting the three study end points. The 10-year ACM model demonstrated the best AUC performance (0.72; per-year mortality of 2.6% above and 0.8% below the risk threshold), followed by 2-year ACM (0.71; per-year mortality of 1.13% above and 0.3% below the risk threshold) and prediction of extrapulmonary significant incidental findings (0.70; probability of occurrence of 25.4% above and 9.6% below the threshold). Conclusion A fully automated AI model indicated extrapulmonary structures at risk on chest CT scans and predicted ACM with explanations. ClinicalTrials.gov Identifier: NCT00047385 © RSNA, 2024 Supplemental material is available for this article. See also the editorial by Yanagawa and Hata in this issue.
Conflict of interest statement
Figures





References
-
- Computed tomography (CT) exams (indicator). OECD. https://www.oecd.org/en/data/indicators/computed-tomography-ct-exams.html. Published 2021. Accessed November 17, 2023.
-
- Census 2020 Redistricting Data (Public Law 94-171) Summary Files. U.S . Census Bureau . https://www.census.gov/programs-surveys/decennial-census/about/rdo/summa.... Published 2023. Accessed November 17, 2023 .
-
- Pinsky PF , Dunn B , Gierada D , et al . Incidental renal tumours on low-dose CT lung cancer screening exams . J Med Screen 2017. ; 24 ( 2 ): 104 – 109 . - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

