EFFICACY AND SAFETY OF THE PROPOSED BIOSIMILAR AFLIBERCEPT, SDZ-AFL, IN PATIENTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: 52-Week Results From the Phase 3 Mylight Study
- PMID: 39287533
- PMCID: PMC11398290
- DOI: 10.1097/IAE.0000000000004174
EFFICACY AND SAFETY OF THE PROPOSED BIOSIMILAR AFLIBERCEPT, SDZ-AFL, IN PATIENTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: 52-Week Results From the Phase 3 Mylight Study
Abstract
Purpose: The Phase 3 Mylight study was designed to confirm clinical equivalence of proposed biosimilar aflibercept (SOK583A1; Sandoz [proposed biosimilar aflibercept, SDZ-AFL]) to its reference biologic (Eylea; Regeneron Pharmaceuticals, Inc; Bayer AG [reference aflibercept, Ref-AFL]).
Method: Mylight was a prospective, double-masked, 2-arm, parallel Phase 3 study. Participants with neovascular age-related macular degeneration were randomized 1:1 to receive eight injections of SDZ-AFL (n = 244) or Ref-AFL (n = 240) over 48 weeks. The primary endpoint was mean change in best-corrected visual acuity score from baseline to Week 8. Secondary endpoints included anatomical outcomes, best-corrected visual acuity at Weeks 24 and 52, safety, and pharmacokinetics.
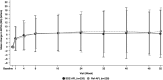
Results: Similarity in mean change in best-corrected visual acuity score was established between SDZ-AFL (n = 235) and Ref-AFL (n = 226) at Week 8 (difference: -0.3 [90% CI, -1.5 to 1.0]) and Week 52. No clinically meaningful differences occurred between groups in anatomical outcomes. Safety profiles were similar, with comparable incidences of treatment-related adverse events (SDZ-AFL: 2.5%; Ref-AFL: 2.9%). The incidence of anti-drug antibodies was similar between groups. Systemic free aflibercept concentrations 24 hours postdose were low and comparable between SDZ-AFL and Ref-AFL.
Conclusion: Proposed biosimilar aflibercept matched reference aflibercept in efficacy, safety, and pharmacokinetics in participants with neovascular age-related macular degeneration. Therefore, this Phase 3 study confirmed biosimilarity of SDZ-AFL to Ref-AFL.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Opthalmic Communications Society, Inc.
Figures




References
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

