Pilot implementation of the revised criteria for staging of Alzheimer's disease by the Alzheimer's Association Workgroup in a tertiary memory clinic
- PMID: 39287564
- PMCID: PMC11567817
- DOI: 10.1002/alz.14245
Pilot implementation of the revised criteria for staging of Alzheimer's disease by the Alzheimer's Association Workgroup in a tertiary memory clinic
Abstract
Introduction: We aimed to evaluate the feasibility of the 2024 Alzheimer's Association Workgroup's integrated clinical-biological staging scheme in outpatient settings within a tertiary memory clinic.
Methods: The 2018 syndromal cognitive staging system, coupled with a binary biomarker classification, was implemented for 236 outpatients with cognitive concerns. The 2024 numeric clinical staging framework, incorporating biomarker staging, was specifically applied to 154 individuals within the Alzheimer's disease (AD) continuum.
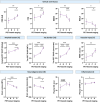
Results: The 2024 staging scheme accurately classified 95.5% AD. Among these, 56.5% exhibited concordant clinical and biological stages (canonical), 34.7% demonstrated more advanced clinical stages than biologically expected (susceptible), and 8.8% displayed the inverse pattern (resilient). The susceptible group was characterized by a higher burden of neurodegeneration and inflammation than anticipated from tau, whereas the resilient group showed the opposite.
Discussion: The 2024 staging scheme is generally feasible. A discrepancy between clinical and biological stages is relatively frequent among symptomatic patients with AD.
Highlights: The 2024 AA staging scheme is generally feasible in a tertiary memory clinic. A discrepancy between clinical and biological stages is relatively frequent in AD. The mismatch may be influenced by a non-specific pathological process involved in AD. Individual profiles like aging and lifestyles may contribute to such a mismatch. Matched and mismatched cases converge toward similar clinical outcomes.
Keywords: Alzheimer's disease; amyloid; clinical‐biological staging scheme; inflammation; neurodegeneration; positron emission tomography; tau.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Tzu‐Chen Yen is an employee of APRINOIA Therapeutics Co., Ltd. (Suzhou, China). All other authors have no conflicts of interest to declare. Author disclosures are available in the supporting information.
Figures



References
-
- Therriault J, Schindler SE, Salvadó G, et al. Biomarker‐based staging of Alzheimer disease: rationale and clinical applications. Nat Rev Neurol. 2024;20:232‐244. - PubMed
-
- Ding D, Zhao Q, Guo Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross‐sectional analysis of the Shanghai Aging Study. Alzheimer's Dement. 2015;11:300‐309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

