Drug-drug interactions of icenticaftor (QBW251) with a 5-probe cytochrome P450 cocktail and oral contraceptives
- PMID: 39288032
- PMCID: PMC11407298
- DOI: 10.1111/cts.70028
Drug-drug interactions of icenticaftor (QBW251) with a 5-probe cytochrome P450 cocktail and oral contraceptives
Abstract
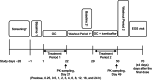
A drug-drug interaction (DDI) study was conducted to evaluate the effect of icenticaftor (QBW251) on the pharmacokinetics (PK) of a 5-probe cytochrome P450 (CYP) substrate cocktail, guided by in vitro studies in human hepatocytes and liver microsomes. Another DDI study investigated the effect of icenticaftor on the PK and pharmacodynamics (PD) of a monophasic oral contraceptive (OC) containing ethinyl estradiol (EE) and levonorgestrel (LVG) in premenopausal healthy female subjects. The static-mechanistic DDI assessment indicated that icenticaftor may moderately induce the metabolic clearance of co-medications metabolized by CYP3A4 (area under the concentration-time curve [AUC] ratio: 0.47) and potentially CYP2C; icenticaftor may also weakly inhibit the metabolic clearance of co-medications metabolized by CYP1A2 and CYP3A4 (AUC ratio: 1.35 and 1.86, respectively) and moderately inhibit CYP2B6 (AUC ratio: 2.11). In the CYP substrate cocktail DDI study, icenticaftor 300 mg twice daily (b.i.d.) moderately inhibited CYP1A2 (AUC ratio: 3.35) and CYP2C19 (AUC ratio: 2.70). As expected from the results of the in vitro studies, weak induction was observed for CYP3A4 (AUC ratio: 0.51) and CYP2C8 (AUC ratio: 0.66). In the OC DDI study, co-administration of icenticaftor 450 mg b.i.d. with monophasic OC containing 30-μg EE and 150-μg LVG once daily reduced the plasma exposure of both components by approximately 50% and led to increased levels of follicle-stimulating hormone and luteinizing hormone. These results provide valuable guidance for the use of icenticaftor in patients taking concomitant medications that are substrates of CYP enzymes or patients using OCs.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of Novartis. All authors except LB hold Novartis shares. The authors declared no other competing interests for this work.
Figures



References
-
- Glaenzel U, Huth F, Eggimann F, et al. Absorption, distribution, metabolism, and excretion of icenticaftor (QBW251) in healthy male volunteers at steady state and in vitro phenotyping of major metabolites. Drug Metab Disp 2024, accepted on 6th Sep 2024.
-
- US Food and Drug Administration . Clinical drug interaction studies — cytochrome P450 enzyme‐ and transporter‐mediated drug interactions: guidance for industry. 2020. Accessed October, 2023. https://www.fda.gov/media/134581/download
-
- European Medicines Agency . Guideline on the investigation of drug interactions: CPMP/EWP/560/95/Rev. 1 Corr. 2. 2012. Accessed October, 2023. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐in...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

