Effect of a plant extract of fenugreek (Trigonella foenum-graecum) on testosterone in blood plasma and saliva in a double blind randomized controlled intervention study
- PMID: 39288153
- PMCID: PMC11407615
- DOI: 10.1371/journal.pone.0310170
Effect of a plant extract of fenugreek (Trigonella foenum-graecum) on testosterone in blood plasma and saliva in a double blind randomized controlled intervention study
Abstract
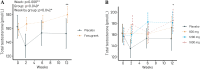
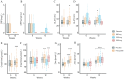
Many aging men experience reduced energy and libido related to non-optimal testosterone levels. We conducted a randomized double-blind trial with TrigozimR fenugreek extract to assess impact on plasma and saliva testosterone, and some subjective effects. 95 men (40-80y) completed a 12-week intervention, taking 3 tablets daily with 0 mg (placebo; n = 22), 600 mg (n = 21), 1200 mg (n = 25) and1800 mg (n = 27) fenugreek extract and essential nutrients. Samples were collected at weeks 0, 2, 6, and 12. Participants answered a pre- and post-intervention questionnaire on lifestyle and libido. We measured total testosterone (HPLC-MS/MS) and sex hormone binding globulin (ELISA), calculated free testosterone index (FTI), and measured saliva testosterone. Plasma total testosterone and FTI increased after any dose of TrigozimR vs. baseline (13.0%, p = 1.0x10-4 and 16.3%, p = 6.2x10-6), but not vs. placebo (9.0%, p = 0.122 and 11.3% p = 0.059). 1800 mg TrigozimR resulted in 12.2% increased FTI (p = 0.025). Saliva testosterone concentration increased after any dose of TrigozimR vs. baseline (31.1%, p = 2.3x10-4) and vs. placebo (37.2%, p = 0.042). 1800 mg TrigozimR for 12 weeks resulted in 19.6% (p = 0.006) increased saliva testosterone. Compliance was confirmed by enhanced plasma concentration of 25-hydroxy vitamin D3. We observed no subjective effects or side-effects of TrigozimR. TrigozimR increased saliva and plasma testosterone concentration during intervention but only for saliva vs. placebo. Saliva may be preferred for measuring free testosterone due to no protein-bound testosterone.
Copyright: © 2024 Lee-Ødegård et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
TEG is CEO and stockowner in Vitas Ltd. CAD is stockowner, consultant, and board member in the contract laboratory Vitas Ltd where the intervention was performed and where most of the laboratory analyses were carried out. CAD is CEO and stockowner in the consulting company DBG Ltd responsible for design and execution. We received funding from the commercial source Vitas Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials. SL was paid for performing the statistical analyses.
Figures



References
-
- Guo Y, Maharjan S, Narsingh R, Shrestha S, Upadhyaya A, Nepal P, et al. Furosap, a novel fenugreek seed extract improves lean body mass and serum testosterone in a randomized, placebo-controlled, double-blind clinical investigation. Funct Foods Health Dis. 2018;8(11):519–30. doi: 10.31989/ffhd.v8i11.565 - DOI
-
- Rao A, Steels E, Beccaria G, Inder WJ, Vitetta L. Testofen, a specialised Trigonella foenum-graecum seed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. 2016;19:134–42. doi: 10.3109/13685538.2015.1135323 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

