Conformational ensembles in Klebsiella pneumoniae FimH impact uropathogenesis
- PMID: 39288182
- PMCID: PMC11441496
- DOI: 10.1073/pnas.2409655121
Conformational ensembles in Klebsiella pneumoniae FimH impact uropathogenesis
Abstract
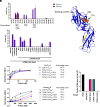
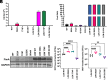
Klebsiella pneumoniae is an important pathogen causing difficult-to-treat urinary tract infections (UTIs). Over 1.5 million women per year suffer from recurrent UTI, reducing quality of life and causing substantial morbidity and mortality, especially in the hospital setting. Uropathogenic E. coli (UPEC) is the most prevalent cause of UTI. Like UPEC, K. pneumoniae relies on type 1 pili, tipped with the mannose-binding adhesin FimH, to cause cystitis. However, K. pneumoniae FimH is a poor binder of mannose, despite a mannose-binding pocket identical to UPEC FimH. FimH is composed of two domains that are in an equilibrium between tense (low-affinity) and relaxed (high-affinity) conformations. Substantial interdomain interactions in the tense conformation yield a low-affinity, deformed mannose-binding pocket, while domain-domain interactions are broken in the relaxed state, resulting in a high-affinity binding pocket. Using crystallography, we identified the structural basis by which domain-domain interactions direct the conformational equilibrium of K. pneumoniae FimH, which is strongly shifted toward the low-affinity tense state. Removal of the pilin domain restores mannose binding to the lectin domain, thus showing that poor mannose binding by K. pneumoniae FimH is not an inherent feature of the mannose-binding pocket. Phylogenetic analyses of K. pneumoniae genomes found that FimH sequences are highly conserved. However, we surveyed a collection of K. pneumoniae isolates from patients with long-term indwelling catheters and identified isolates that possessed relaxed higher-binding FimH variants, which increased K. pneumoniae fitness in bladder infection models, suggesting that long-term residence within the urinary tract may select for higher-binding FimH variants.
Keywords: UTI; bacterial pathogenesis; mechanism of bacterial colonization.
Conflict of interest statement
Competing interests statement:D.A.H. serves on the Board of Directors of BioVersys AG, Basel, Switzerland. S.J.H., J.W.J., and J.S.P. own company stock in Fimbrion Therapeutics, who has licensed the mannoside patents, and they may benefit if the company is successful in marketing mannosides, S.J.H., J.W.J. and J.S.P. are co-inventors on the issued patents US 10,273,260, US 9,957,289, and US 8,937,167 which cover the use of mannoside-based FimH ligand antagonists for the treatment of disease. J.W.J. is an inventor on the patent applications covering FIM1006, FIM1028, FIM1033, and FIM2065; WO2017156508 and US20200002303.
Figures



References
-
- Foxman B., Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North Am. 28, 1–13 (2014). - PubMed
-
- De Lastours V., Foxman B., Urinary tract infection in diabetes: Epidemiologic considerations topical collection on genitourinary infections. Curr. Infect Dis. Rep. 16, 1–6 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

