CRISPR/Cas13d targeting suppresses repeat-associated non-AUG translation of C9orf72 hexanucleotide repeat RNA
- PMID: 39288267
- PMCID: PMC11527445
- DOI: 10.1172/JCI179016
CRISPR/Cas13d targeting suppresses repeat-associated non-AUG translation of C9orf72 hexanucleotide repeat RNA
Abstract
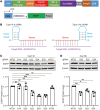
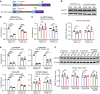
A hexanucleotide GGGGCC repeat expansion in the non-coding region of the C9orf72 gene is the most common genetic mutation identified in patients with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The resulting repeat RNA and dipeptide repeat proteins from non-conventional repeat translation have been recognized as important markers associated with the diseases. CRISPR/Cas13d, a powerful RNA-targeting tool, has faced challenges in effectively targeting RNA with stable secondary structures. Here we report that CRISPR/Cas13d can be optimized to specifically target GGGGCC repeat RNA. Our results demonstrate that the CRISPR/Cas13d system can be harnessed to significantly diminish the translation of poly-dipeptides originating from the GGGGCC repeat RNA. This efficacy has been validated in various cell types, including induced pluripotent stem cells and differentiated motor neurons originating from C9orf72-ALS patients, as well as in C9orf72 repeat transgenic mice. These findings demonstrate the application of CRISPR/Cas13d in targeting RNA with intricate higher-order structures and suggest a potential therapeutic approach for ALS and FTD.
Keywords: Gene therapy; Genetics; Molecular biology; Neurodegeneration; Neuroscience.
Conflict of interest statement
HL and JW are listed as inventors on a patent application (application number: 63313150) filed by Johns Hopkins University on technologies related to this manuscript.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

