Management of low-level HIV viremia during antiretroviral therapy: Delphi consensus statement and appraisal of the evidence
- PMID: 39288982
- PMCID: PMC11503133
- DOI: 10.1136/sextrans-2024-056199
Management of low-level HIV viremia during antiretroviral therapy: Delphi consensus statement and appraisal of the evidence
Abstract
Objective: While antiretroviral therapy (ART) is highly effective, detection of low levels of HIV-1 RNA in plasma is common in treated individuals. Given the uncertainties on the topic, we convened a panel of experts to consider different clinical scenarios, producing a Delphi consensus to help guide clinical practice.
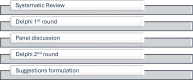
Methods: A panel of 17 experts in infectious diseases, virology and immunology rated 32 statements related to four distinct scenarios: (1) low-level viremia during stable (≥6 months) first-line ART (≥2 consecutive HIV-1 RNA measurements 50-500 copies/mL); (2) a viral blip during otherwise suppressive ART (a HIV-1 RNA measurement 50-1000 copies/mL with adjacent measurements <50 copies/mL); (3) low-level viral rebound during previously suppressive ART (≥2 consecutive HIV-1 RNA measurements 50-500 copies/mL); (4) residual viremia during suppressive ART (persistent HIV-1 RNA quantification below 50 copies/mL). A systematic review, conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement, informed the 32 statements. The Delphi procedure was modified to include two voting rounds separated by a moderated group discussion. Grading of Recommendations, Assessment, Development, and Evaluations-based recommendations were developed.
Results: Overall, 18/32 statements (56.2%) achieved a strong consensus, 3/32 (9.4%) achieved a moderate consensus and 11/32 (34.4%) did not achieve a consensus. Across the four scenarios, the panel unanimously emphasised the importance of implementing specific interventions prior to considering therapy changes, including assessing adherence, testing for genotypic drug resistance and scheduling more frequent follow-up visits. Strategies indicated in selected circumstances included therapeutic drug monitoring, quantifying total HIV-1 DNA and evaluating concomitant chronic infections.
Conclusions: While acknowledging the many uncertainties about source, significance and optimal management of low-level viremia during ART, the findings provide insights to help harmonise clinical practice. There is a need for well-designed randomised studies assessing different interventions to manage low-level viremia and future research regarding its definition.
Keywords: Anti-HIV Agents; Guidelines as Topic; HIV.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures