Iron chelation as a new therapeutic approach to prevent senescence and liver fibrosis progression
- PMID: 39289337
- PMCID: PMC11408630
- DOI: 10.1038/s41419-024-07063-0
Iron chelation as a new therapeutic approach to prevent senescence and liver fibrosis progression
Abstract
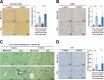
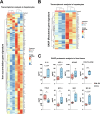
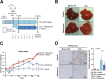
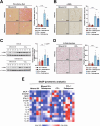
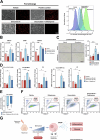
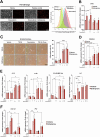
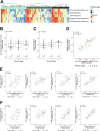
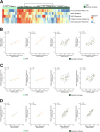
Iron overload and cellular senescence have been implicated in liver fibrosis, but their possible mechanistic connection has not been explored. To address this, we have delved into the role of iron and senescence in an experimental model of chronic liver injury, analyzing whether an iron chelator would prevent liver fibrosis by decreasing hepatocyte senescence. The model of carbon tetrachloride (CCl4) in mice was used as an experimental model of liver fibrosis. Results demonstrated that during the progression of liver fibrosis, accumulation of iron occurs, concomitant with the appearance of fibrotic areas and cells undergoing senescence. Isolated parenchymal hepatocytes from CCl4-treated mice present a gene transcriptomic signature compatible with iron accumulation and senescence, which correlates with induction of Reactive Oxygen Species (ROS)-related genes, activation of the Transforming Growth Factor-beta (TGF-β) pathway and inhibition of oxidative metabolism. Analysis of the iron-related gene signature in a published single-cell RNA-seq dataset from CCl4-treated livers showed iron accumulation correlating with senescence in other non-parenchymal liver cells. Treatment with deferiprone, an iron chelator, attenuated iron accumulation, fibrosis and senescence, concomitant with relevant changes in the senescent-associated secretome (SASP), which switched toward a more anti-inflammatory profile of cytokines. In vitro experiments in human hepatocyte HH4 cells demonstrated that iron accumulates in response to a senescence-inducing reagent, doxorubicin, being deferiprone able to prevent senescence and SASP, attenuating growth arrest and cell death. However, deferiprone did not significantly affect senescence induced by two different agents (doxorubicin and deoxycholic acid) or activation markers in human hepatic stellate LX-2 cells. Transcriptomic data from patients with different etiologies demonstrated the relevance of iron accumulation in the progression of liver chronic damage and fibrosis, correlating with a SASP-related gene signature and pivotal hallmarks of fibrotic changes. Altogether, our study establishes iron accumulation as a clinically exploitable driver to attenuate pathological senescence in hepatocytes.
© 2024. The Author(s).
Conflict of interest statement
MS is shareholder of Senolytic Therapeutics, Inc., Life Biosciences, Inc., Rejuveron Senescence Therapeutics, AG, and Altos Labs, Inc.
Figures