Quantifiable blood TCR repertoire components associate with immune aging
- PMID: 39289351
- PMCID: PMC11408526
- DOI: 10.1038/s41467-024-52522-z
Quantifiable blood TCR repertoire components associate with immune aging
Abstract
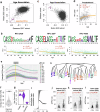
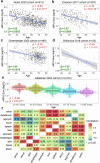
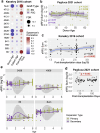
T cell senescence alters the homeostasis of distinct T cell populations and results in decayed adaptive immune protection in older individuals, but a link between aging and dynamic T cell clone changes has not been made. Here, using a newly developed computational framework, Repertoire Functional Units (RFU), we investigate over 6500 publicly available TCR repertoire sequencing samples from multiple human cohorts and identify age-associated RFUs consistently across different cohorts. Quantification of RFU reduction with aging reveals accelerated loss under immunosuppressive conditions. Systematic analysis of age-associated RFUs in clinical samples manifests a potential link between these RFUs and improved clinical outcomes, such as lower ICU admission and reduced risk of complications, during acute viral infections. Finally, patients receiving bone marrow transplantation show a secondary expansion of the age-associated clones upon stem cell transfer from younger donors. Together, our results suggest the existence of a 'TCR clock' that could reflect the immune functions in aging populations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

