Sensing data and methodology from the Adaptive DBS Algorithm for Personalized Therapy in Parkinson's Disease (ADAPT-PD) clinical trial
- PMID: 39289373
- PMCID: PMC11408616
- DOI: 10.1038/s41531-024-00772-5
Sensing data and methodology from the Adaptive DBS Algorithm for Personalized Therapy in Parkinson's Disease (ADAPT-PD) clinical trial
Abstract
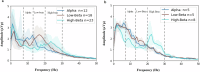
Adaptive deep brain stimulation (aDBS) is an emerging advancement in DBS technology; however, local field potential (LFP) signal rate detection sufficient for aDBS algorithms and the methods to set-up aDBS have yet to be defined. Here we summarize sensing data and aDBS programming steps associated with the ongoing Adaptive DBS Algorithm for Personalized Therapy in Parkinson's Disease (ADAPT-PD) pivotal trial (NCT04547712). Sixty-eight patients were enrolled with either subthalamic nucleus or globus pallidus internus DBS leads connected to a Medtronic PerceptTM PC neurostimulator. During the enrollment and screening procedures, a LFP (8-30 Hz, ≥1.2 µVp) control signal was identified by clinicians in 84.8% of patients on medication (65% bilateral signal), and in 92% of patients off medication (78% bilateral signal). The ADAPT-PD trial sensing data indicate a high LFP signal presence in both on and off medication states of these patients, with bilateral signal in the majority, regardless of PD phenotype.
© 2024. The Author(s).
Conflict of interest statement
This study is funded by Medtronic and employees of Medtronic have been involved in the conceptualization, design, data collection and analysis, and preparation of the manuscript as it is a pivotal trial of adaptive deep brain stimulation. S.S., R.S., L.T., Y.T., R.R., M.C., and N.M. are employees of Medtronic and may hold shares and/or stock options in the company. All investigators and their institutions received sponsorship for the conduct of the ADAPT-PD clinical trial from Medtronic. J.O. reports research support from Medtronic and Boston Scientific, and consulting payments from Abbott. L.A. has received honoraria from educational and advisory board consulting work for Medtronic and Boston Scientific. K.M. reports research support from Medtronic, Boston Scientific, and Surgical Information Sciences and consulting payments from Medtronic and Boston Scientific. T.H. reports research support from Medtronic and Boston Scientific and consulting payments from Medtronic. E.M. reports research support from Abbott and consulting payments from Abbott and Medtronic. E.M. is Associate Editor of
Figures






References
-
- Limousin, P. & Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol.15, 234–242 (2019). - PubMed
-
- Fasano, A., Aquino, C. C., Krauss, J. K., Honey, C. R. & Bloem, B. R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol.11, 98–110 (2015). - PubMed
-
- Fox, S. H. et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord.33, 1248–1266 (2018). - PubMed
-
- Schuepbach, W. M. M. et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med368, 610–622 (2013). - PubMed

