Phase 1 dose-escalation trial evaluating a group 2 influenza hemagglutinin stabilized stem nanoparticle vaccine
- PMID: 39289377
- PMCID: PMC11408684
- DOI: 10.1038/s41541-024-00959-0
Phase 1 dose-escalation trial evaluating a group 2 influenza hemagglutinin stabilized stem nanoparticle vaccine
Abstract
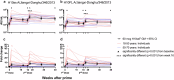
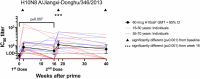
The relative conservation of the influenza hemagglutinin (HA) stem compared to that of the immunodominant HA head makes the HA stem an attractive target for broadly protective influenza vaccines. Here we report the first-in-human, dose-escalation, open-label trial (NCT04579250) evaluating an unadjuvanted group 2 stabilized stem ferritin nanoparticle vaccine based on the H10 A/Jiangxi-Donghu/346/2013 influenza HA, H10ssF, in healthy adults. Participants received a single 20 mcg dose (n = 3) or two 60 mcg doses 16 weeks apart (n = 22). Vaccination with H10ssF was safe and well tolerated with only mild systemic and local reactogenicity reported. No serious adverse events occurred. Vaccination significantly increased homologous H10 HA stem binding and neutralizing antibodies at 2 weeks after both first and second vaccinations, and these responses remained above baseline at 40 weeks. Heterologous H3 and H7 binding antibodies also significantly increased after each vaccination and remained elevated throughout the study. These data indicate that the group 2 HA stem nanoparticle vaccine is safe and induces stem-directed binding and neutralizing antibodies.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
B.S.G., J.R.M., and M.K. are inventors on a NIAID/NIH patent application covering the Group 2 influenza stabilized stem. All other authors declare no competing interests.
Figures

References
-
- Centers for Disease Control and Prevention. Disease Burden of Flu. https://www.cdc.gov/flu/about/burden/index.html (2024).
LinkOut - more resources
Full Text Sources

