Prediction of early clinical response to neoadjuvant chemotherapy in Triple-negative breast cancer: Incorporating Radiomics through breast MRI
- PMID: 39289507
- PMCID: PMC11408492
- DOI: 10.1038/s41598-024-72581-y
Prediction of early clinical response to neoadjuvant chemotherapy in Triple-negative breast cancer: Incorporating Radiomics through breast MRI
Abstract
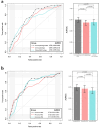
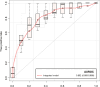
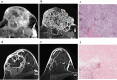
This study assessed pretreatment breast MRI coupled with machine learning for predicting early clinical responses to neoadjuvant chemotherapy (NAC) in triple-negative breast cancer (TNBC), focusing on identifying non-responders. A retrospective analysis of 135 TNBC patients (107 responders, 28 non-responders) treated with NAC from January 2015 to October 2022 was conducted. Non-responders were defined according to RECIST guidelines. Data included clinicopathologic factors and clinical MRI findings, with radiomics features from contrast-enhanced T1-weighted images, to train a stacking ensemble of 13 machine learning models. For subgroup analysis, propensity score matching was conducted to adjust for clinical disparities in NAC response. The efficacy of the models was evaluated using the area under the receiver-operating-characteristic curve (AUROC) before and after matching. The model combining clinicopathologic factors and clinical MRI findings achieved an AUROC of 0.752 (95% CI 0.644-0.860) for predicting non-responders, while radiomics-based models showed 0.749 (95% CI 0.614-0.884). An integrated model of radiomics, clinicopathologic factors, and clinical MRI findings reached an AUROC of 0.802 (95% CI 0.699-0.905). After propensity score matching, the hierarchical order of key radiomics features remained consistent. Our study demonstrated the potential of using machine learning models based on pretreatment MRI to non-invasively predict TNBC non-responders to NAC.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing conflicts of interest to disclose.
Figures

References
-
- Hatzis, C. et al. Relationship between complete pathologic response to neoadjuvant chemotherapy and survival in triple-negative breast cancer. Clin. Cancer Res.22, 26–33 (2016). - PubMed
-
- Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. The Lancet384, 164–172 (2014). - PubMed
-
- Haque, W. et al. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res. Treat.170, 559–567 (2018). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

