Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Arba Minch Zuria District, Gamo Zone, Southwest Ethiopia
- PMID: 39289715
- PMCID: PMC11406784
- DOI: 10.1186/s12936-024-05087-7
Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Arba Minch Zuria District, Gamo Zone, Southwest Ethiopia
Abstract
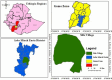
Background: Artemether-lumefantrine (AL) has been the primary anti-malarial drug used to treat uncomplicated Plasmodium falciparum malaria in Ethiopia since 2004. However, there have been recent reports of AL resistance mutations in different African countries, including Ethiopia. This is concerning and requires periodic monitoring of anti-malarial drug resistance. Therefore, the current study aimed to evaluate the therapeutic efficacy of AL in treating uncomplicated P. falciparum malaria in the Arba Minch Zuria District, Gamo Zone, Southwest Ethiopia.
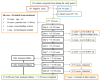
Methods: A single-arm prospective study with a 28-day follow-up period was conducted from July to October 2022. Capillary blood samples were collected for RDT and microscopic examination. The study enrolled monoinfected P. falciparum patients aged ≥ 18 years at Ganta Sira Health Post. Sociodemographic and clinical data were recorded, and a dried blood spot (DBS) was prepared for each participant. Nested polymerase chain reaction (nPCR) genotyping of the msp-1 and msp-2 genes was only performed for recurrent cases to distinguish between recurrence and reinfection. Data entry and analysis were performed using the WHO Excel spreadsheet and SPSS version 26.
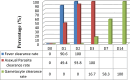
Results: A total of 89 patients were enrolled, and 67 adequately completed the 28-day follow-up period. AL showed a 100% clearance rate for fever on day 2 and asexual parasites on day 3. Gametocytes were detected in 13.5% (12/89) of the participants. The gametocyte clearance rate was 58.3% (7/12) until day 7 and 100% (12/12) until day 14. Five participants developed recurrent malaria, three of whom experienced relapse and two of whom experienced reinfection. Based on the Kaplan-Meier survival analysis, the PCR-uncorrected and PCR-corrected cumulative incidence of success were 93.7% (95% CI 85.5-97.3) and 96.2% (95% CI 85.5-98.7), respectively.
Conclusion: AL was efficacious in treating uncomplicated P. falciparum malaria in the study area. However, the detection of recurrent patients highlights the need for continuous efficacy studies in this area.
Keywords: Cure rate; Malaria; Parasite clearance; Recurrence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Chewaka District, Ethiopia.Malar J. 2020 Jul 10;19(1):240. doi: 10.1186/s12936-020-03307-4. Malar J. 2020. PMID: 32650784 Free PMC article.
-
Increasing day three parasitaemia is observed after treatment of patients with artemether-lumefantrine and single dose of primaquine for uncomplicated Plasmodium falciparum malaria in Arbaminch Zuria district, Southwest Ethiopia.Malar J. 2025 Mar 18;24(1):89. doi: 10.1186/s12936-025-05337-2. Malar J. 2025. PMID: 40102850 Free PMC article.
-
Therapeutic efficacy and safety of artemether-lumefantrine combination therapy for the treatment of uncomplicated Plasmodium falciparum malaria at Teda Health Centre, Northwest Ethiopia, 2022/23.Malar J. 2024 Aug 30;23(1):266. doi: 10.1186/s12936-024-05082-y. Malar J. 2024. PMID: 39215366 Free PMC article.
-
Efficacy and safety of artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ethiopia: a systematic review and meta-analysis.Malar J. 2021 May 6;20(1):213. doi: 10.1186/s12936-021-03745-8. Malar J. 2021. PMID: 33957925 Free PMC article.
-
Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD004529. doi: 10.1002/14651858.CD004529.pub3. Cochrane Database Syst Rev. 2021. PMID: 33459345 Free PMC article.
Cited by
-
Artemisinin resistant kelch13 R622I and RDT negativity approaching predominance in northern Ethiopia and emerging C580Y of African origin threaten falciparum malaria control.medRxiv [Preprint]. 2025 Jun 23:2025.06.23.25330019. doi: 10.1101/2025.06.23.25330019. medRxiv. 2025. PMID: 40666313 Free PMC article. Preprint.
-
Rising prevalence of Plasmodium falciparum Artemisinin partial resistance mutations in Ethiopia.Commun Med (Lond). 2025 Jul 18;5(1):297. doi: 10.1038/s43856-025-01008-0. Commun Med (Lond). 2025. PMID: 40681807 Free PMC article.
References
-
- WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
-
- U.S. President’s Malaria Initiative. Ethiopia Malaria Operational Plan FY. 2023
-
- Federal Democratic Republic of Ethiopia Ministry of Health. Ethiopia malaria elimination strategic plan: 2021–2025. Ethiopia: Addis Ababa; 2020
-
- WHO. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva: World Health Organization; 2010.
-
- WHO. Guidelines for malaria – 2021. Geneva: World Health Organization; 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

