Clinical efficacy and safety of faecal microbiota transplantation in the treatment of irritable bowel syndrome: a systematic review, meta-analysis and trial sequential analysis
- PMID: 39289768
- PMCID: PMC11409544
- DOI: 10.1186/s40001-024-02046-5
Clinical efficacy and safety of faecal microbiota transplantation in the treatment of irritable bowel syndrome: a systematic review, meta-analysis and trial sequential analysis
Abstract
Background: The aim of this study is to evaluate the efficacy and safety of faecal microbiota transplantation (FMT) for the treatment of irritable bowel syndrome (IBS).
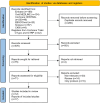
Methods: We searched four databases for randomised controlled trials (RCTs) that compared FMT with a control intervention in patients with IBS. The revised Cochrane risk-of-bias (RoB) tool was chosen for appraisal. Meta-analysis with trial sequential analysis (TSA) was conducted. Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology was used to assess the certainty of evidence (CoE).
Results: We included 12 RCTs with a total of 615 participants. Meta-analyses showed no significant difference between the FMT and control groups in terms of clinical responses (relative risk [RR] = 1.44, 95% confidence interval [CI] 0.88-2.33) and changes in IBS Severity Scoring System (IBS-SSS) scores (standardised mean difference [SMD] = - 0.31, 95% CI - 0.72 to 0.09) and IBS Quality of Life (IBS-QOL) scores (SMD = 0.30, 95% CI - 0.09 to 0.69). Subgroup analysis revealed that in studies with low RoB and using endoscopy, nasojejunal tube and rectal enema delivery, FMT led to a significant improvement in clinical responses and changes in IBS-SSS and IBS-QOL scores. TSA suggested that the current evidence is inconclusive and that the CoE is very low.
Conclusion: This study suggests that patients with IBS may benefit from FMT especially when it is administered via endoscopy, nasojejunal tube or rectal enema. However, the certainty of evidence is very low. Further research is needed to confirm the efficacy and safety of FMT for IBS treatment.
Trial registration: PROSPERO registration number CRD42020211002.
Keywords: Faecal microbiota transplantation; Irritable bowel syndrome; Meta-analysis; Randomised controlled trial; Systemic review.
© 2024. The Author(s).
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures




References
-
- Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407. - PubMed
-
- Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66(6):1075–82. - PubMed
-
- Glynn E, Galambosi G, Boland K. Resolution of weight loss, systemic illness, and fever after triple therapy: a surprising response to treatment of positive urease test. Gastroenterology. 2021;160(5):e1–3. - PubMed
-
- Staley C, Khoruts A, Sadowsky MJ. Contemporary applications of fecal microbiota transplantation to treat intestinal diseases in humans. Arch Med Res. 2017;48(8):766–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

