Copper homeostasis and cuproptosis in health and disease
- PMID: 39290254
- PMCID: PMC11406047
- DOI: 10.1002/mco2.724
Copper homeostasis and cuproptosis in health and disease
Abstract
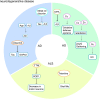
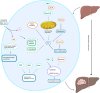
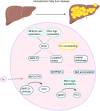
Copper is a vital trace element in human physiology, essential for the synthesis of numerous crucial metabolic enzymes and facilitation of various biological processes. Regulation of copper levels within a narrow range is imperative for maintaining metabolic homeostasis. Numerous studies have demonstrated the significant roles of copper homeostasis and cuproptosis in health and disease pathogenesis. However, a comprehensive and up-to-date systematic review in this domain remains absent. This review aims to consolidate recent advancements in understanding the roles of cuproptosis and copper homeostasis in health and disease, focusing on the underlying mechanisms and potential therapeutic interventions. Dysregulation of copper homeostasis, manifesting as either copper excess or deficiency, is implicated in the etiology of various diseases. Cuproptosis, a recently identified form of cell death, is characterized by intracellular copper overload. This phenomenon mediates a diverse array of evolutionary processes in organisms, spanning from health to disease, and is implicated in genetic disorders, liver diseases, neurodegenerative disorders, and various cancers. This review provides a comprehensive summary of the pathogenic mechanisms underlying cuproptosis and copper homeostasis, along with associated targeted therapeutic agents. Furthermore, it explores future research directions with the potential to yield significant advancements in disease treatment, health management, and disease prevention.
Keywords: cancer; cardiovascular disease; copper homeostasis; cuproptosis; hereditary disease; liver disease; neurodegenerative disease.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
