Asymmetric Dirhodium-Catalyzed Modification of Immunomodulatory Imide Drugs and Their Biological Assessment
- PMID: 39291008
- PMCID: PMC11403733
- DOI: 10.1021/acsmedchemlett.4c00297
Asymmetric Dirhodium-Catalyzed Modification of Immunomodulatory Imide Drugs and Their Biological Assessment
Abstract
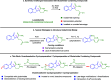
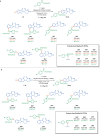
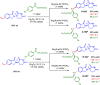
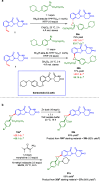
Cereblon (CRBN) has been successfully co-opted to affect the targeted degradation of "undruggable" proteins with immunomodulatory imide drugs (IMiDs). IMiDs act as molecule glues that facilitate ternary complex formation between CRBN and a target protein, leading to ubiquitination and proteasomal degradation. Subtle structural modifications often cause profound and sometimes unpredictable changes in the degradation selectivity. Herein, we successfully utilize enantioselective cyclopropanation and cyclopropenation on intact glutarimides to enable the preparation of stereochemically and regiochemically matched molecular pairs for structure-activity relationship (SAR) analysis across several classical CRBN neosubstrates. The resulting glutarimide analogs were found to reside in unique chemical space when compared to other IMiDs in the public domain. SAR studies revealed that, in addition to the more precedented impacts of regiochemistry, stereochemical modifications far from the glutarimide can lead to divergent neosubstrate selectivity. These findings emphasize the importance of enabling enantioselective methods for glutarimide-containing compounds to tune the degradation selectivity.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): HMLD is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued March 10, 2015).
Figures

References
-
- D’Amato R. J.; Loughnan M. S.; Flynn E.; Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4082–4085. 10.1073/pnas.91.9.4082. - DOI - PMC - PubMed
- Sampaio E. P.; Sarno E. N.; Galilly R.; Cohn Z. A.; Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 1991, 173, 699–703. 10.1084/jem.173.3.699. - DOI - PMC - PubMed
-
- Krönke J.; Udeshi N. D.; Narla A.; Grauman P.; Hurst S. N.; McConkey M.; Svinkina T.; Heckl D.; Comer E.; Li X.; Ciarlo C.; Hartman E.; Munshi N.; Schenone M.; Schreiber S. L.; Carr S. A.; Ebert B. L. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science 2014, 343, 301–305. 10.1126/science.1244851. - DOI - PMC - PubMed
- Lu G.; Middleton R. E.; Sun H.; Naniong M.; Ott C. J.; Mitsiades C. S.; Wong K.-K.; Bradner J. E.; Kaelin W. G. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science 2014, 343, 305–309. 10.1126/science.1244917. - DOI - PMC - PubMed
-
- Petzold G.; Fischer E. S.; Thomä N. H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase. Nature 2016, 532, 127–130. 10.1038/nature16979. - DOI - PubMed
- Matyskiela M. E.; Lu G.; Ito T.; Pagarigan B.; Lu C.-C.; Miller K.; Fang W.; Wang N.-Y.; Nguyen D.; Houston J.; Carmel G.; Tran T.; Riley M.; Nosaka L. A.; Lander G. C.; Gaidarova S.; Xu S.; Ruchelman A. L.; Handa H.; Carmichael J.; Daniel T. O.; Cathers B. E.; Lopez-Girona A.; Chamberlain P. P. A novel cereblon modulator recruits GSPT1 to the CRL4CRBN ubiquitin ligase. Nature 2016, 535, 252–257. 10.1038/nature18611. - DOI - PubMed
- Sievers Q. L.; Petzold G.; Bunker R. D.; Renneville A.; Słabicki M.; Liddicoat B. J.; Abdulrahman W.; Mikkelsen T.; Ebert B. L.; Thomä N. H. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 2018, 362, eaat0572 10.1126/science.aat0572. - DOI - PMC - PubMed
- Matyskiela M. E.; Clayton T.; Zheng X.; Mayne C.; Tran E.; Carpenter A.; Pagarigan B.; McDonald J.; Rolfe M.; Hamann L. G.; Lu G.; Chamberlain P. P. Crystal structure of the SALL4–pomalidomide–cereblon–DDB1 complex. Nat. Struct. Mol. Biol. 2020, 27, 319–322. 10.1038/s41594-020-0405-9. - DOI - PubMed
- Wang E. S.; Verano A. L.; Nowak R. P.; Yuan J. C.; Donovan K. A.; Eleuteri N. A.; Yue H.; Ngo K. H.; Lizotte P. H.; Gokhale P. C.; Gray N. S.; Fischer E. S. Acute pharmacological degradation of Helios destabilizes regulatory T cells. Nat. Chem. Biol. 2021, 17, 711–717. 10.1038/s41589-021-00802-w. - DOI - PMC - PubMed
- Watson E. R.; Novick S.; Matyskiela M. E.; Chamberlain P. P.; e la Peña A. H.; Zhu J.; Tran E.; Griffin P. R.; Wertz I. E.; Lander G. C. Molecular glue CELMoD compounds are regulators of cereblon conformation. Science 2022, 378, 549–553. 10.1126/science.add7574. - DOI - PMC - PubMed
- Bonazzi S.; d’Hennezel E.; Beckwith R. E. J.; Xu L.; Fazal A.; Magracheva A.; Ramesh R.; Cernijenko A.; Antonakos B.; Bhang H.-e. C.; Caro R. G.; Cobb J. S.; Ornelas E.; Ma X.; Wartchow C. A.; Clifton M. C.; Forseth R. R.; Fortnam B. H.; Lu H.; Csibi A.; Tullai J.; Carbonneau S.; Thomsen N. M.; Larrow J.; Chie-Leon B.; Hainzl D.; Gu Y.; Lu D.; Meyer M. J.; Alexander D.; Kinyamu-Akunda J.; Sabatos-Peyton C. A.; Dales N. A.; Zécri F. J.; Jain R. K.; Shulok J.; Wang Y. K.; Briner K.; Porter J. A.; Tallarico J. A.; Engelman J. A.; Dranoff G.; Bradner J. E.; Visser M.; Solomon J. M. Discovery and characterization of a selective IKZF2 glue degrader for cancer immunotherapy. Cell Chem. Biol. 2023, 30, 235–247. 10.1016/j.chembiol.2023.02.005. - DOI - PubMed
-
- Yamanaka S.; Furihata H.; Yanagihara Y.; Taya A.; Nagasaka T.; Usui M.; Nagaoka K.; Shoya Y.; Nishino K.; Yoshida S.; Kosako H.; Tanokura M.; Miyakawa T.; Imai Y.; Shibata N.; Sawasaki T. Lenalidomide derivatives and proteolysis-targeting chimeras for controlling neosubstrate degradation. Nat. Commun. 2023, 14, 4683. 10.1038/s41467-023-40385-9. - DOI - PMC - PubMed
- Nowak R. P.; Che J.; Ferrao S.; Kong N. R.; Liu H.; Zerfas B. L.; Jones L. H. Structural rationalization of GSPT1 and IKZF1 degradation by thalidomide molecular glue derivatives. RSC Med. Chem. 2023, 14, 501–506. 10.1039/D2MD00347C. - DOI - PMC - PubMed
- Oleinikovas V.; Gainza P.; Ryckmans T.; Fasching B.; Thomä N. H. From Thalidomide to Rational Molecular Glue Design for Targeted Protein Degradation. Annu. Rev. Pharmacool. Toxicol. 2024, 64, 291–312. 10.1146/annurev-pharmtox-022123-104147. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

