Colchicine treatment in amyotrophic lateral sclerosis: safety, biological and clinical effects in a randomized clinical trial
- PMID: 39291166
- PMCID: PMC11406549
- DOI: 10.1093/braincomms/fcae304
Colchicine treatment in amyotrophic lateral sclerosis: safety, biological and clinical effects in a randomized clinical trial
Abstract
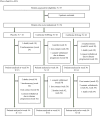
In preclinical studies, the anti-inflammatory drug colchicine, which has never been tested in amyotrophic lateral sclerosis, enhanced the expression of autophagy factors and inhibited accumulation of transactive response DNA-binding protein 43 kDa, a known histopathological marker of amyotrophic lateral sclerosis. This multicentre, randomized, double-blind trial enrolled patients with probable or definite amyotrophic lateral sclerosis who experienced symptom onset within the past 18 months. Patients were randomly assigned in a 1:1:1 ratio to receive colchicine at a dose of 0.005 mg/kg/day, 0.01 mg/kg/day or placebo for a treatment period of 30 weeks. The number of positive responders, defined as patients with a decrease lesser than 4 points in the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised total score during the 30-week treatment period, was the primary outcome. Disease progression, survival, safety and quality of life at the end of treatment were the secondary clinical outcomes. Secondary biological outcomes included changes from baseline to treatment end of stress granule and autophagy responses, transactive response DNA-binding protein 43 kDa, neurofilament accumulation and extracellular vesicle secretion, between the colchicine and placebo groups. Fifty-four patients were randomized to receive colchicine (n = 18 for each colchicine arm) or placebo (n = 18). The number of positive responders did not differ between the placebo and colchicine groups: 2 out of 18 patients (11.1%) in the placebo group, 5 out of 18 patients (27.8%) in the colchicine 0.005 mg/kg/day group (odds ratio = 3.1, 97.5% confidence interval 0.4-37.2, P = 0.22) and 1 out of 18 patients (5.6%) in the colchicine 0.01 mg/kg/day group (odds ratio = 0.5, 97.5% confidence interval 0.01-10.2, P = 0.55). During treatment, a slower Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised decline was detected in patients receiving colchicine 0.005 mg/kg/day (mean difference = 0.53, 97.5% confidence interval 0.07-0.99, P = 0.011). Eight patients experienced adverse events in placebo arm (44.4%), three in colchicine 0.005 mg/kg/day (16.7%) and seven in colchicine 0.01 mg/kg/day arm (35.9%). The differences in adverse events were not statistically significant. In conclusion, colchicine treatment was safe for amyotrophic lateral sclerosis patients. Further studies are required to better understand mechanisms of action and clinical effects of colchicine in this condition.
Keywords: amyotrophic lateral sclerosis; colchicine; neuroinflammation; protein quality control; randomized clinical trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.M. reports receiving advisory board fees from Biogen, Amylyx and Italfarmaco, grant support from Roche and grant support from Pfizer (RAP-ALS study; drug furniture); all are not related to the present study. R.D.A., V.C., S.C., V.B., O.P., C.C., G.G., E.Z., R.C.C., I.M., C.S., N.F., R.V., F.T., C.P., N.T., L.D., G.F., A.C., E.D.B., E.D.E., E.S., L.P., A.D., A.P., F.A., V.G. and E.C. declare no competing interests. Disclosure forms provided by the authors are available with the full text of this article.
Figures


References
-
- Mandrioli J, Mediani L, Alberti S, Carra S. ALS and FTD: Where RNA metabolism meets protein quality control. Semin Cell Dev Biol. 2020;99:183–192. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
