Phase 1b/2a Study Assessing the Safety and Efficacy of Felzartamab in Anti-Phospholipase A2 Receptor Autoantibody-Positive Primary Membranous Nephropathy
- PMID: 39291206
- PMCID: PMC11403052
- DOI: 10.1016/j.ekir.2024.06.018
Phase 1b/2a Study Assessing the Safety and Efficacy of Felzartamab in Anti-Phospholipase A2 Receptor Autoantibody-Positive Primary Membranous Nephropathy
Abstract
Introduction: Primary membranous nephropathy (PMN) is most often caused by autoantibodies to phospholipase A2 receptor (PLA2R). M-PLACE (NCT04145440) is an open-label, phase 1b/2a study that assessed the safety and efficacy of the fully human anti-CD38 monoclonal antibody felzartamab in high-risk anti-PLA2R+ PMN.
Methods: Patients with newly diagnosed or relapsed PMN (cohort 1 [C1]; n = 18) or PMN refractory to immunosuppressive therapy (IST) (cohort 2 [C2]; n = 13) received 9 infusions of felzartamab 16 mg/kg in the 24-week treatment period, followed by a 28-week follow-up. The primary end point was the incidence and severity of treatment-emergent adverse events (TEAEs).
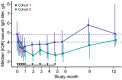
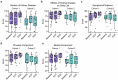
Results: A total of 31 patients were enrolled and received felzartamab. Twenty-seven patients (87.1%) had TEAEs, including infusion-related reactions (IRRs) (29.0%), hypogammaglobulinemia (25.8%), peripheral edema (19.4%), and nausea (16.1%). Five patients (16.1%) had serious TEAEs that all resolved. Immunologic response (anti-PLA2R titer reduction ≥50%) was achieved by 20 of 26 efficacy-evaluable patients (76.9%) (C1, 13/15 [86.7%]; C2, 7/11 [63.6%]). Anti-PLA2R titer reductions were rapid (week 1 response, 44.0%; response 7 months after last felzartamab dose [end of study, EOS], 53.8%). Partial proteinuria remission (urine protein-to-creatinine ratio [UPCR] reduction ≥50%, UPCR <3.0 g/g, and stable estimated glomerular filtration rate [eGFR]) was achieved by 9 of 26 patients (34.6%) (C1, 7/15 [46.7%]; C2, 2/11 [18.2%]) before or at EOS (median follow-up, 366 days). Serum albumin increased from baseline to EOS in 20 of 26 patients (76.9%) (C1, 12/15 [80.0%]; C2, 8/11 [72.7%]).
Conclusion: In this population with high-risk anti-PLA2R+ PMN, felzartamab was tolerated and resulted in rapid partial and complete immunologic responses and partial improvements in proteinuria and serum albumin in some patients.
Keywords: MOR202; clinical trial; felzartamab; phase 2; phospholipase A2 receptor; primary membranous nephropathy.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

