Lung epithelial-endothelial-mesenchymal signaling network with hepatocyte growth factor as a hub is involved in bronchopulmonary dysplasia
- PMID: 39291265
- PMCID: PMC11405311
- DOI: 10.3389/fcell.2024.1462841
Lung epithelial-endothelial-mesenchymal signaling network with hepatocyte growth factor as a hub is involved in bronchopulmonary dysplasia
Abstract
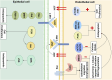
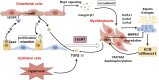
Bronchopulmonary dysplasia (BPD) is fundamentally characterized by the arrest of lung development and abnormal repair mechanisms, which result in impaired development of the alveoli and microvasculature. Hepatocyte growth factor (HGF), secreted by pulmonary mesenchymal and endothelial cells, plays a pivotal role in the promotion of epithelial and endothelial cell proliferation, branching morphogenesis, angiogenesis, and alveolarization. HGF exerts its beneficial effects on pulmonary vascular development and alveolar simplification primarily through two pivotal pathways: the stimulation of neovascularization, thereby enriching the pulmonary microvascular network, and the inhibition of the epithelial-mesenchymal transition (EMT), which is crucial for maintaining the integrity of the alveolar structure. We discuss HGF and its receptor c-Met, interact with various growth factors throughout the process of lung development and BPD, and form a signaling network with HGF as a hub, which plays the pivotal role in orchestrating and integrating epithelial, endothelial and mesenchymal.
Keywords: angiogenesis; bronchopulmonary dysplasia (BPD); epithelial-mesenchymal transition (EMT); growth factors; hepatocyte growth factor (HGF).
Copyright © 2024 Sang and Qiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung.Am J Physiol Lung Cell Mol Physiol. 2016 Jun 1;310(11):L1098-110. doi: 10.1152/ajplung.00423.2015. Epub 2016 Apr 1. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27036872 Free PMC article.
-
Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Lung Injury in Rat Model of Bronchopulmonary Dysplasia by Affecting Cell Survival and Angiogenesis.Stem Cells Dev. 2020 Dec 1;29(23):1520-1532. doi: 10.1089/scd.2020.0156. Epub 2020 Nov 4. Stem Cells Dev. 2020. PMID: 33040709
-
RhoA-Dependent HGF and c-Met Mediate Gas6-Induced Inhibition of Epithelial-Mesenchymal Transition, Migration, and Invasion of Lung Alveolar Epithelial Cells.Biomolecules. 2019 Oct 4;9(10):565. doi: 10.3390/biom9100565. Biomolecules. 2019. PMID: 31590238 Free PMC article.
-
Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease.Am J Respir Crit Care Med. 2007 May 15;175(10):978-85. doi: 10.1164/rccm.200611-1660PP. Epub 2007 Feb 1. Am J Respir Crit Care Med. 2007. PMID: 17272782 Free PMC article. Review.
-
miR34a: a novel small molecule regulator with a big role in bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L228-L235. doi: 10.1152/ajplung.00279.2020. Epub 2021 Apr 7. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33825492 Review.
Cited by
-
Mechanistic Insights and Therapeutic Potential of Wnt5a Signaling in Alveolar Epithelial Cell Development and Bronchopulmonary Dysplasia.Stem Cell Rev Rep. 2025 Aug 16. doi: 10.1007/s12015-025-10951-3. Online ahead of print. Stem Cell Rev Rep. 2025. PMID: 40817998 Review.
-
Biomarkers associated with the diagnosis and prognosis of Mycoplasma pneumoniae pneumonia in children: a review.Front Cell Infect Microbiol. 2025 Mar 18;15:1552144. doi: 10.3389/fcimb.2025.1552144. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40171163 Free PMC article. Review.
References
-
- Arjaans S., Wagner B. D., Mourani P. M., Mandell E. W., Poindexter B. B., Berger R. M. F., et al. (2020). Early angiogenic proteins associated with high risk for bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Am. J. physiology. Lung Cell. Mol. physiology 318 (4), L644–L654. 10.1152/ajplung.00131.2019 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

