Regulation of TGF-β1-induced fibroblast differentiation of human periodontal ligament stem cells through the mutually antagonistic action of ectonucleotide pyrophosphatase/phosphodiesterase 1 and 2
- PMID: 39291269
- PMCID: PMC11405333
- DOI: 10.3389/fcell.2024.1426762
Regulation of TGF-β1-induced fibroblast differentiation of human periodontal ligament stem cells through the mutually antagonistic action of ectonucleotide pyrophosphatase/phosphodiesterase 1 and 2
Abstract
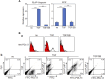
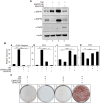
Human periodontal ligament stem cells (hPDLSCs) differentiate into periodontal ligament (PDL) fibroblasts, osteoblasts, and cementoblasts. To identify inducers of PDL fibroblastic differentiation, monoclonal antibody series were developed a series of against membrane/extracellular matrix (ECM) molecules through decoy immunization. The anti-PDL13 antibody targets ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), renowned for regulating skeletal and soft tissue mineralization. ENPP1 accumulates in the periodontal ligament region of tooth roots, and specifically localizes to the cell boundaries and elongated processes of the fibroblastic cells. As ENPP1 expression increases during fibroblastic differentiation, mineralization induced by tissue-nonspecific alkaline phosphatase (TNAP), a pyrophosphate-degrading enzyme, is completely inhibited. This is consistent with ENPP1 and TNAP acting in opposition, and TGF-β1-induced ENPP1 expression creates an essential environment for PDL fibroblast differentiation. Representative fibroblastic differentiation markers decrease with endogenous ENPP1 inhibition by siRNA and antibody blocking. ENPP2 generates lipid signaling molecules. In contrast to ENPP1, ENPP2 disappears in TGF-β1-induced PDL fibroblasts. Ectopic expression of ENPP2 hinders TGF-β1-induced PDL fibroblastic differentiation. Suppression of ENPP1 and ENPP2 leads to severe defects in undifferentiated and differentiated cells, demonstrating that these two factors play opposing roles in soft and hard tissue differentiation but can complement each other for cell survival. In conclusion, increased ENPP1 is crucial for TGF-β1-induced PDL differentiation, while ENPP2 and TNAP can inhibit ENPP1. ENPP1 and ENPP2 exhibit complementary functions in the cell survival.
Keywords: ENPP1; ENPP2; TGF-β1; fibroblastic differentiation; periodontal ligament stem cells (PDLSC).
Copyright © 2024 Ju, Ko and Jang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Addison W. N., Azari F., Sorensen E. S., Kaartinen M. T., McKee M. D. (2007). Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 282 (21), 15872–15883. 10.1074/jbc.M701116200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

