Selective Oxidation of Methanol to Methyl Formate on Gold: The Role of Low-Coordinated Sites Revealed by Isothermal Pulsed Molecular Beam Experiments and AIMD Simulations
- PMID: 39291271
- PMCID: PMC11403654
- DOI: 10.1021/acs.jpcc.4c03959
Selective Oxidation of Methanol to Methyl Formate on Gold: The Role of Low-Coordinated Sites Revealed by Isothermal Pulsed Molecular Beam Experiments and AIMD Simulations
Abstract
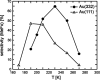
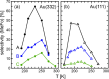
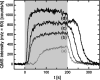
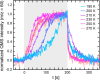
To elucidate the role of low-coordinated sites in the partial methanol oxidation to methyl formate (MeFo), the isothermal reactivity of flat Au(111) and stepped Au(332) in pulsed molecular beam experiments was compared for a broad range of reaction conditions. Low-coordinated step sites were found to enhance MeFo selectivity, especially at low coverage conditions, as found at higher temperatures. The analysis of the transient kinetics provides evidence for the essential role of Au x O y phases for MeFo formation and the complex interplay of different oxygen species for the observed selectivity. Ab initio molecular dynamic simulations yielded microscopic insights in the formation of Au x O y phases on flat and stepped gold surfaces emphasizing the role of low-coordinated sites in their formation. Moreover, associated surface restructuring provides atomic-scale insights which align with the experimentally observed transient kinetics in MeFo formation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Unraveling the Role of Water in Isothermal Methanol Partial Oxidation to Methyl Formate on Gold: A Combined Experimental and Computational Study.J Phys Chem C Nanomater Interfaces. 2025 Jan 10;129(3):1611-1626. doi: 10.1021/acs.jpcc.4c05968. eCollection 2025 Jan 23. J Phys Chem C Nanomater Interfaces. 2025. PMID: 39877428 Free PMC article.
-
Partial Oxidation of Methanol on Gold: How Selectivity Is Steered by Low-Coordinated Sites.ACS Catal. 2024 May 6;14(10):7901-7906. doi: 10.1021/acscatal.3c04578. eCollection 2024 May 17. ACS Catal. 2024. PMID: 38779185 Free PMC article.
-
Methanol oxidation on Au(332): an isothermal pulsed molecular beam study.Phys Chem Chem Phys. 2021 Oct 6;23(38):21599-21605. doi: 10.1039/d1cp03436g. Phys Chem Chem Phys. 2021. PMID: 34558565
-
Heterogeneity of oxygen reactivity: key for selectivity of partial methanol oxidation on gold surfaces.Chem Commun (Camb). 2022 Mar 31;58(27):4336-4339. doi: 10.1039/d1cc06939j. Chem Commun (Camb). 2022. PMID: 35290427
-
Ag/Au mixed sites promote oxidative coupling of methanol on the alloy surface.Chemistry. 2014 Apr 14;20(16):4646-52. doi: 10.1002/chem.201304837. Epub 2014 Mar 13. Chemistry. 2014. PMID: 24633724
Cited by
-
Unraveling the Role of Water in Isothermal Methanol Partial Oxidation to Methyl Formate on Gold: A Combined Experimental and Computational Study.J Phys Chem C Nanomater Interfaces. 2025 Jan 10;129(3):1611-1626. doi: 10.1021/acs.jpcc.4c05968. eCollection 2025 Jan 23. J Phys Chem C Nanomater Interfaces. 2025. PMID: 39877428 Free PMC article.
References
-
- Kaiser D.; Beckmann L.; Walter J.; Bertau M. Conversion of Green Methanol to Methyl Formate. Catalysts 2021, 11 (7), 869.10.3390/catal11070869. - DOI
-
- Lee J. S.; Kim J. C.; Kim Y. G. Methyl formate as a new building block in C1 chemistry. Appl. Catal. 1990, 57 (1), 1–30. 10.1016/S0166-9834(00)80720-4. - DOI
-
- Klezl P.Treibstoff für Verbrennungsmotoren und Verwendung von Methylformiat. EP 0501097 B1, 1995.
-
- Rong L.; Xu Z.; Sun J.; Guo G. New methyl formate synthesis method: Coal to methyl formate. J. Energy Chem. 2018, 27 (1), 238–242. 10.1016/j.jechem.2017.07.015. - DOI
-
- Sang R.; Wei Z. H.; Hu Y. Y.; Alberico E.; Wei D.; Tian X. X.; Ryabchuk P.; Spannenberg A.; Razzaq R.; Jackstell R.; et al. Methyl formate as a hydrogen energy carrier. Nat. Catal. 2023, 6 (6), 543–550. 10.1038/s41929-023-00959-8. - DOI
LinkOut - more resources
Full Text Sources
