Ligand-to-Metal Charge Transfer (LMCT) Catalysis: Harnessing Simple Cerium Catalysts for Selective Functionalization of Inert C-H and C-C Bonds
- PMID: 39291873
- PMCID: PMC11669090
- DOI: 10.1021/acs.accounts.4c00510
Ligand-to-Metal Charge Transfer (LMCT) Catalysis: Harnessing Simple Cerium Catalysts for Selective Functionalization of Inert C-H and C-C Bonds
Abstract
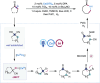
ConspectusChemists have long pursued harnessing light energy and photoexcitation processes for synthetic transformations. Ligand-to-metal charge transfer (LMCT) in high-valent metal complexes often triggers bond homolysis, generating oxidized ligand-centered radicals and reduced metal centers. While photoinduced oxidative activations can be enabled, this process, typically seen as photochemical decomposition, remains underexplored in catalytic applications. To mitigate decomposition during LMCT excitation, we developed a catalytic cycle integrating in situ coordination, LMCT, and ligand homolysis to activate ligated alcohols transiently into alkoxy radicals. This catalytic approach leverages Ce(IV) LMCT excitation and highly reactive alkoxy radical intermediates for selective functionalizations of C(sp3)-H and C(sp3)-C(sp3) bonds under mild conditions. In this Account, we discuss these advancements, highlighting the practical utility of cost-effective cerium salts as catalysts and their potential to develop innovative transformations, addressing long-standing synthetic challenges.Selective functionalization of chemically inert C(sp3)-H bonds has long posed a significant challenge. We first detail our research using LMCT-enabled alkoxy radical-mediated hydrogen atom transfer (HAT) processes for selective C(sp3)-H functionalizations. Using readily available CeCl3, we established a general protocol for employing free alcohols in the Barton reaction. By integrating LMCT and HAT catalysis, we introduced a selective photocatalytic strategy for functionalizing feedstock alkanes, converting gaseous hydrocarbons into valuable products. Employing simple cerium salts like Ce(OTf)3 and CeCl3, we achieved selective C-H amination of methane and ethane at ambient temperature, achieving turnover numbers of 2900 and 9700, respectively. This catalytic manifold has been further exploited to address the site-selectivity challenge in the C-H functionalization of linear alkanes. The use of methanol as a cocatalyst enabled preferential functionalization of the most electron-rich sites, achieving a high intrinsic selectivity over 12:1 of secondary vs primary sites in pentane and hexane.Next, we discuss the catalytic utilization of alkoxy-radical-mediated β-scission, a frequently encountered side reaction in HAT transformations, for selective cleavage and functionalization of C-C bonds. The versatility of the LMCT catalytic platform facilitates the generation of alkoxy radicals from various free alcohols. In our initial demonstration of LMCT-enabled C(sp3)-C(sp3) bond activation, we developed a cerium-catalyzed ring-opening and amination of cycloalkanols, providing an effective protocol for cleaving unstrained C-C bonds. This strategy has been successfully applied to various radical cross-coupling processes, leading to innovative transformations such as ring expansions of cycloalkanols, dehydroxymethylative alkylation, amination, alkenylation, and ring expansions of cyclic ketones. These results highlight the synthetic potential of employing LMCT-mediated β-scission and ubiquitous C-C bonds as unconventional functional handles for generating molecular complexity.Lastly, we delve into our mechanistic investigations. Beyond the catalytic application of Ce(IV) LMCT in various transformations, we have undertaken comprehensive mechanistic studies. These investigations encompass characterization of Ce(IV) alkoxide complexes to elucidate their structures, evaluation of their photoactivity and selectivity in radical generation, and elucidation of kinetic pathways associated with transient LMCT excited states. Our research has revealed ultrafast bond homolysis, back electron transfer, and the selectivity of heteroleptic complexes in homolysis, providing crucial insights for advancing LMCT catalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










Similar articles
-
Cerium-Catalyzed C-H Functionalizations of Alkanes Utilizing Alcohols as Hydrogen Atom Transfer Agents.J Am Chem Soc. 2020 Apr 1;142(13):6216-6226. doi: 10.1021/jacs.0c00212. Epub 2020 Mar 23. J Am Chem Soc. 2020. PMID: 32181657
-
Identification of Alkoxy Radicals as Hydrogen Atom Transfer Agents in Ce-Catalyzed C-H Functionalization.J Am Chem Soc. 2023 Jan 11;145(1):359-376. doi: 10.1021/jacs.2c10126. Epub 2022 Dec 20. J Am Chem Soc. 2023. PMID: 36538367
-
Asymmetric Catalytic Radical Reactions Enabled by Chiral N,N'-Dioxide-Metal Complexes.Acc Chem Res. 2025 Aug 5;58(15):2496-2510. doi: 10.1021/acs.accounts.5c00370. Epub 2025 Jul 12. Acc Chem Res. 2025. PMID: 40650579
-
LMCT-Driven Iron Photocatalysis: Mechanistic Insights and Synthetic Applications.Chemistry. 2025 Sep 10:e02185. doi: 10.1002/chem.202502185. Online ahead of print. Chemistry. 2025. PMID: 40926633 Review.
-
N-Heterocyclic Carbene Enabled Functionalization of Inert C(Sp3)-H Bonds via Hydrogen Atom Transfer (HAT) Processes.Chemistry. 2024 Aug 22;30(47):e202401811. doi: 10.1002/chem.202401811. Epub 2024 Aug 2. Chemistry. 2024. PMID: 39092881 Review.
Cited by
-
Iron-Catalyzed Aerobic Carbonylation of Methane via Ligand-to-Metal Charge Transfer Excitation.J Am Chem Soc. 2025 Jan 15;147(2):1440-1447. doi: 10.1021/jacs.4c16449. Epub 2025 Jan 6. J Am Chem Soc. 2025. PMID: 39760382 Free PMC article.
-
Light-Driven C(sp3)-C(sp3) Bond Functionalizations Enabled by the PCET Activation of Alcohol O-H Bonds.Acc Chem Res. 2025 Jul 1;58(13):2061-2071. doi: 10.1021/acs.accounts.5c00246. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40511721
-
Unlocking Chromium Decarboxylative Ligand-to-Metal Charge Transfer: Efficient and Redox-Neutral Allylation of Aldehydes Using Carboxylic Acids.J Am Chem Soc. 2025 Jul 2;147(26):22759-22767. doi: 10.1021/jacs.5c04691. Epub 2025 Jun 16. J Am Chem Soc. 2025. PMID: 40518937 Free PMC article.
-
Catalysis in the Excited State: Bringing Innate Transition Metal Photochemistry into Play.ACS Catal. 2025 Mar 5;15(6):4665-4680. doi: 10.1021/acscatal.4c07962. eCollection 2025 Mar 21. ACS Catal. 2025. PMID: 40144674 Free PMC article. Review.
-
Dehomologative C-C Borylation of Aldehydes and Alcohols via a Rh-Catalyzed Dehydroformylation-Borylation Relay.J Am Chem Soc. 2025 May 21;147(20):16735-16741. doi: 10.1021/jacs.5c02181. Epub 2025 May 12. J Am Chem Soc. 2025. PMID: 40354369 Free PMC article.
References
-
- An Q.; Xing Y.-Y.; Pu R.; Jia M.; Chen Y.; Hu A.; Zhang S.-Q.; Yu N.; Du J.; Zhang Y.; Chen J.; Liu W.; Hong X.; Zuo Z. Identification of Alkoxy Radicals as Hydrogen Atom Transfer Agents in Ce-Catalyzed C-H Functionalization. J. Am. Chem. Soc. 2023, 145, 359–376. 10.1021/jacs.2c10126. - DOI - PubMed
LinkOut - more resources
Full Text Sources

