Frequent and asymmetric cell division in endosymbiotic bacteria of cockroaches
- PMID: 39291985
- PMCID: PMC11497835
- DOI: 10.1128/aem.01466-24
Frequent and asymmetric cell division in endosymbiotic bacteria of cockroaches
Abstract
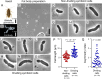
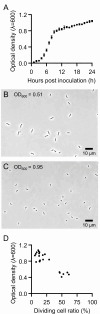
Many insects are obligatorily associated with and dependent on specific microbial species as essential mutualistic partners. In the host insects, such microbial mutualists are usually maintained in specialized cells or organs, called bacteriocytes or symbiotic organs. Hence, potentially exponential microbial growth cannot be realized but must be strongly constrained by spatial and resource limitations within the host cells or tissues. How such endosymbiotic bacteria grow, divide, and proliferate is important for understanding the interactions and dynamics underpinning intimate host-microbe symbiotic associations. Here we report that Blattabacterium, the ancient and essential endosymbiont of cockroaches, exhibits unexpectedly high rates of cell division (20%-58%) and, in addition, the cell division is asymmetric (average asymmetry index >1.5) when isolated from the German cockroach Blattella germanica. The asymmetric division of endosymbiont cells at high frequencies was observed irrespective of host tissues (fat bodies vs ovaries) or developmental stages (adults vs nymphs vs embryos) of B. germanica, and also observed in several different cockroach species. By contrast, such asymmetric and frequent cell division was observed neither in Buchnera, the obligatory bacterial endosymbiont of aphids, nor in Pantoea, the obligatory bacterial gut symbiont of stinkbugs. Comparative genomics of cell division-related genes uncovered that the Blattabacterium genome lacks the Min system genes that determine the cell division plane, which may be relevant to asymmetric cell division. These observations combined with comparative symbiont genomics provide insight into what processes and regulations may underpin the growth, division, and proliferation of such bacterial mutualists continuously constrained under within-host conditions.IMPORTANCEDiverse insects are dependent on specific bacterial mutualists for their survival and reproduction. Due to the long-lasting coevolutionary history, such symbiotic bacteria tend to exhibit degenerative genomes and suffer uncultivability. Because of their microbiological fastidiousness, the cell division patterns of such uncultivable symbiotic bacteria have been poorly described. Here, using fine microscopic and quantitative morphometric approaches, we report that, although bacterial cell division usually proceeds through symmetric binary fission, Blattabacterium, the ancient and essential endosymbiont of cockroaches, exhibits frequent and asymmetric cell division. Such peculiar cell division patterns were not observed with other uncultivable essential symbiotic bacteria of aphids and stinkbugs. Gene repertoire analysis revealed that the molecular machinery for regulating the bacterial cell division plane are lost in the Blattabacterium genome, suggesting the possibility that the general trend toward the reductive genome evolution of symbiotic bacteria may underpin their bizarre cytological/morphological traits.
Keywords: Blattabacterium; Blattella germanica; Buchnera; Escherichia coli; Pantoea; aphid; cell division; cockroach; stinkbug; symbiotic bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Grants and funding
- JPMJER1902/MEXT | Japan Science and Technology Agency (JST)
- JPMJER1902/MEXT | Japan Science and Technology Agency (JST)
- JPMJER1902/MEXT | Japan Science and Technology Agency (JST)
- JP24H02294/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K08935/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources

