Manipulating mitochondrial reactive oxygen species alters survival in unexpected ways in a Drosophila Cdk5 model of neurodegeneration
- PMID: 39292114
- PMCID: PMC11552616
- DOI: 10.1242/bio.060515
Manipulating mitochondrial reactive oxygen species alters survival in unexpected ways in a Drosophila Cdk5 model of neurodegeneration
Abstract
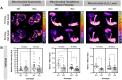
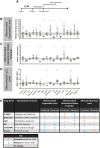
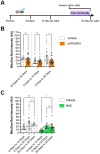
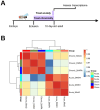
Reactive oxygen species (ROS) are associated with aging and neurodegeneration, but the significance of this association remains obscure. Here, using a Drosophila Cdk5 model of age-related neurodegeneration, we probe this relationship in the pathologically relevant tissue, the brain, by quantifying three specific mitochondrial ROS and manipulating these redox species pharmacologically. Our goal is to ask whether pathology-associated changes in redox state are detrimental for survival, whether they may be beneficial responses to pathology, or whether they are covariates of pathology that do not alter viability. We find, surprisingly, that increasing mitochondrial H2O2 correlates with improved survival. We also find evidence that drugs that alter the mitochondrial glutathione redox potential modulate survival primarily through the compensatory effects they induce rather than through their direct effects on the final mitochondrial glutathione redox potential. We also find that the response to treatment with a redox-altering drug varies depending on the age and genotype of the individual receiving the drug as well as the duration of the treatment. These data have important implications for the design and interpretation of studies investigating the effect of redox state on health and disease as well as on efforts to modify the redox state to achieve therapeutic goals.
Keywords: Cdk5; Mitochondria; Neurodegeneration; Reactive oxygen species (ROS).
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Update of
-
MANIPULATING MITOCHONDRIAL REACTIVE OXYGEN SPECIES ALTERS SURVIVAL IN UNEXPECTED WAYS IN A DROSOPHILA MODEL OF NEURODEGENERATION.bioRxiv [Preprint]. 2024 Mar 29:2024.03.25.586603. doi: 10.1101/2024.03.25.586603. bioRxiv. 2024. Update in: Biol Open. 2024 Jul 15;13(10):bio060515. doi: 10.1242/bio.060515. PMID: 38585927 Free PMC article. Updated. Preprint.
Similar articles
-
MANIPULATING MITOCHONDRIAL REACTIVE OXYGEN SPECIES ALTERS SURVIVAL IN UNEXPECTED WAYS IN A DROSOPHILA MODEL OF NEURODEGENERATION.bioRxiv [Preprint]. 2024 Mar 29:2024.03.25.586603. doi: 10.1101/2024.03.25.586603. bioRxiv. 2024. Update in: Biol Open. 2024 Jul 15;13(10):bio060515. doi: 10.1242/bio.060515. PMID: 38585927 Free PMC article. Updated. Preprint.
-
Changes in mitochondrial distribution occur at the axon initial segment in association with neurodegeneration in Drosophila.Biol Open. 2024 Jul 15;13(7):bio060335. doi: 10.1242/bio.060335. Epub 2024 Jul 11. Biol Open. 2024. PMID: 38912559 Free PMC article.
-
Measuring Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium in Drosophila Neuron Subtypes Using Redox-Sensitive Fluorophores and 3D Imaging.Methods Mol Biol. 2021;2276:113-127. doi: 10.1007/978-1-0716-1266-8_8. Methods Mol Biol. 2021. PMID: 34060036
-
Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria.Redox Biol. 2016 Aug;8:110-8. doi: 10.1016/j.redox.2015.12.010. Epub 2015 Dec 31. Redox Biol. 2016. PMID: 26773874 Free PMC article. Review.
-
The redox language in neurodegenerative diseases: oxidative post-translational modifications by hydrogen peroxide.Cell Death Dis. 2021 Jan 11;12(1):58. doi: 10.1038/s41419-020-03355-3. Cell Death Dis. 2021. PMID: 33431811 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

