Gut microbiota-derived butyrate selectively interferes with growth of carbapenem-resistant Escherichia coli based on their resistance mechanism
- PMID: 39292563
- PMCID: PMC11529417
- DOI: 10.1080/19490976.2024.2397058
Gut microbiota-derived butyrate selectively interferes with growth of carbapenem-resistant Escherichia coli based on their resistance mechanism
Abstract
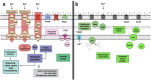
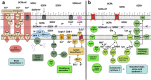
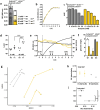
We investigated consequences of resistance acquisition in Escherichia coli clinical isolates during anaerobic (continuous culture) growth and examined their sensitivity to butyrate, a hallmark metabolite of healthy gut microbiota. Strains were stratified based on carrying either a carbapenemase (CARB) or displaying porin malfunctioning (POR). POR displayed markedly altered growth efficiencies, lower membrane stability and increased sensitivity to butyrate compared with CARB. Major differences in global gene expression between the two groups during anaerobic growth were revealed involving increased expression of alternative substrate influx routes, the stringent response and iron acquisition together with lower expression of various stress response systems in POR. Longitudinal analyses during butyrate wash-in showed common responses for all strains as well as specific features of POR that displayed strong initial "overshoot" reactions affecting various stress responses that balanced out over time. Results were partly reproduced in a mutant strain verifying porin deficiencies as the major underlying mechanism for results observed in clinical isolates. Furthermore, direct competition experiments confirmed butyrate as key for amplifying fitness disadvantages based on porin malfunctioning. Results provide new (molecular) insights into ecological consequences of resistance acquisition and can assist in developing measures to prevent colonization and infection based on the underlying resistance mechanism.
Keywords: Antibiotic resistance; Escherichia coli; anaerobic cultivation; bacterial physiology; butyrate; gene expression; gut microbiota.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet [Internet]. 2022;399(10325):629–21. http://www.ncbi.nlm.nih.gov/pubmed/35065702. - PMC - PubMed
-
- World Health Organization . Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. 2021.
-
- Ma J, Song X, Li M, Yu Z, Cheng W, Yu Z, Zhang W, Zhang Y, Shen A, Sun H, et al. Global spread of carbapenem-resistant enterobacteriaceae: epidemiological features, resistance mechanisms, detection and therapy. Microbiol Res [Internet]. 2023;266:127249. http://www.ncbi.nlm.nih.gov/pubmed/36356348. - PubMed
-
- Sader HS, Castanheira M, Kimbrough JH, Kantro V, Mendes RE.. Aztreonam/Avibactam activity against a large collection of carbapenem-resistant enterobacterales (CRE) collected in hospitals from Europe, Asia and Latin America (2019–21). JAC Antimicrob Resist [Internet]. 2023;5(2):dlad032. http://www.ncbi.nlm.nih.gov/pubmed/36968952. - PMC - PubMed
-
- Yuan W, Xu J, Guo L, Chen Y, Gu J, Zhang H, Yang C, Yang Q, Deng S, Zhang L, et al. Clinical risk factors and microbiological and intestinal characteristics of carbapenemase-producing enterobacteriaceae colonization and subsequent infection. Microbiol Spectr [Internet]. 2022;10(6):e0190621. http://www.ncbi.nlm.nih.gov/pubmed/36445086 10.1128/spectrum.01906-21. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
