The TIRS trial: Enrollment procedures and baseline characterization of a pediatric cohort to quantify the epidemiologic impact of targeted indoor residual spraying on Aedes-borne viruses in Merida, Mexico
- PMID: 39292670
- PMCID: PMC11410223
- DOI: 10.1371/journal.pone.0310480
The TIRS trial: Enrollment procedures and baseline characterization of a pediatric cohort to quantify the epidemiologic impact of targeted indoor residual spraying on Aedes-borne viruses in Merida, Mexico
Abstract
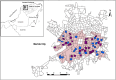
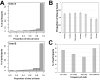
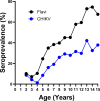
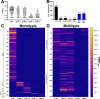
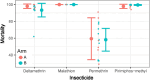
Aedes mosquito-borne viruses (ABVs) place a substantial strain on public health resources in the Americas. Vector control of Aedes mosquitoes is an important public health strategy to decrease or prevent spread of ABVs. The ongoing Targeted Indoor Residual Spraying (TIRS) trial is an NIH-sponsored clinical trial to study the efficacy of a novel, proactive vector control technique to prevent dengue virus (DENV), Zika virus (ZIKV), and chikungunya virus (CHIKV) infections in the endemic city of Merida, Yucatan, Mexico. The primary outcome of the trial is laboratory-confirmed ABV infections in neighborhood clusters. Despite the difficulties caused by the COVID-19 pandemic, by early 2021 the TIRS trial completed enrollment of 4,792 children aged 2-15 years in 50 neighborhood clusters which were allocated to control or intervention arms via a covariate-constrained randomization algorithm. Here, we describe the makeup and ABV seroprevalence of participants and mosquito population characteristics in both arms before TIRS administration. Baseline surveys showed similar distribution of age, sex, and socio-economic factors between the arms. Serum samples from 1,399 children were tested by commercially available ELISAs for presence of anti-ABV antibodies. We found that 45.1% of children were seropositive for one or more flaviviruses and 24.0% were seropositive for CHIKV. Of the flavivirus-positive participants, most were positive for ZIKV-neutralizing antibodies by focus reduction neutralization testing which indicated a higher proportion of participants with previous ZIKV than DENV infections within the cohort. Both study arms had statistically similar seroprevalence for all viruses tested, similar socio-demographic compositions, similar levels of Ae. aegypti infestation, and similar observed mosquito susceptibility to insecticides. These findings describe a population with a high rate of previous exposure to ZIKV and lower titers of neutralizing antibodies against DENV serotypes, suggesting susceptibility to future outbreaks of flaviviruses is possible, but proactive vector control may mitigate these risks.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Yang X, Quam MBM, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. Journal of Travel Medicine. 2021;28(8). - PubMed
-
- Brady OJ, Osgood-Zimmerman A, Kassebaum NJ, Ray SE, de Araújo VEM, da Nóbrega AA, et al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: An observational analysis of over 4 million births. PLOS Medicine. 2019;16(3):e1002755. doi: 10.1371/journal.pmed.1002755 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

