Functional network modules overlap and are linked to interindividual connectome differences during human brain development
- PMID: 39292711
- PMCID: PMC11441662
- DOI: 10.1371/journal.pbio.3002653
Functional network modules overlap and are linked to interindividual connectome differences during human brain development
Abstract
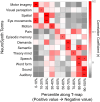
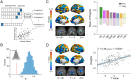
The modular structure of functional connectomes in the human brain undergoes substantial reorganization during development. However, previous studies have implicitly assumed that each region participates in one single module, ignoring the potential spatial overlap between modules. How the overlapping functional modules develop and whether this development is related to gray and white matter features remain unknown. Using longitudinal multimodal structural, functional, and diffusion MRI data from 305 children (aged 6 to 14 years), we investigated the maturation of overlapping modules of functional networks and further revealed their structural associations. An edge-centric network model was used to identify the overlapping modules, and the nodal overlap in module affiliations was quantified using the entropy measure. We showed a regionally heterogeneous spatial topography of the overlapping extent of brain nodes in module affiliations in children, with higher entropy (i.e., more module involvement) in the ventral attention, somatomotor, and subcortical regions and lower entropy (i.e., less module involvement) in the visual and default-mode regions. The overlapping modules developed in a linear, spatially dissociable manner, with decreased entropy (i.e., decreased module involvement) in the dorsomedial prefrontal cortex, ventral prefrontal cortex, and putamen and increased entropy (i.e., increased module involvement) in the parietal lobules and lateral prefrontal cortex. The overlapping modular patterns captured individual brain maturity as characterized by chronological age and were predicted by integrating gray matter morphology and white matter microstructural properties. Our findings highlight the maturation of overlapping functional modules and their structural substrates, thereby advancing our understanding of the principles of connectome development.
Copyright: © 2024 Lei et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Berk LE. Development through the lifespan (7th). London: Pearson; 2017.
-
- Norbom LB, Ferschmann L, Parker N, Agartz I, Andreassen OA, Paus T, et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro-and microstructural MRI findings. Prog Neurobiol. 2021;204:102109. doi: 10.1016/j.pneurobio.2021.102109 . - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

