Lipin1 depletion coordinates neuronal signaling pathways to promote motor and sensory axon regeneration after spinal cord injury
- PMID: 39292743
- PMCID: PMC11441493
- DOI: 10.1073/pnas.2404395121
Lipin1 depletion coordinates neuronal signaling pathways to promote motor and sensory axon regeneration after spinal cord injury
Abstract
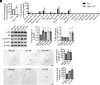
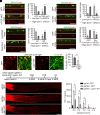
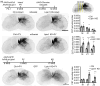
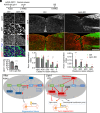
Adult central nervous system (CNS) neurons down-regulate growth programs after injury, leading to persistent regeneration failure. Coordinated lipids metabolism is required to synthesize membrane components during axon regeneration. However, lipids also function as cell signaling molecules. Whether lipid signaling contributes to axon regeneration remains unclear. In this study, we showed that lipin1 orchestrates mechanistic target of rapamycin (mTOR) and STAT3 signaling pathways to determine axon regeneration. We established an mTOR-lipin1-phosphatidic acid/lysophosphatidic acid-mTOR loop that acts as a positive feedback inhibitory signaling, contributing to the persistent suppression of CNS axon regeneration following injury. In addition, lipin1 knockdown (KD) enhances corticospinal tract (CST) sprouting after unilateral pyramidotomy and promotes CST regeneration following complete spinal cord injury (SCI). Furthermore, lipin1 KD enhances sensory axon regeneration after SCI. Overall, our research reveals that lipin1 functions as a central regulator to coordinate mTOR and STAT3 signaling pathways in the CNS neurons and highlights the potential of lipin1 as a promising therapeutic target for promoting the regeneration of motor and sensory axons after SCI.
Keywords: axon regeneration; lipid metabolism; lipid signaling; lipin1; spinal cord injury.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Bradke F., Fawcett J. W., Spira M. E., Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 13, 183–193 (2012). - PubMed
-
- Pfenninger K. H., Plasma membrane expansion: A neuron’s Herculean task. Nat. Rev. Neurosci. 10, 251–261 (2009). - PubMed
-
- Vance J. E., Campenot R. B., Vance D. E., The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim. Biophys. Acta 1486, 84–96 (2000). - PubMed
MeSH terms
Substances
Grants and funding
- AoE/M-604/16 C6034-21G T13-602/21 N 16102519/Hong Kong Research Grant Council
- ITCPD/17-9/Innovation and Technology Commission (ITC)
- 06173946/Hong Kong Center for Neurodegenerative Diseases InnoHK of Hong Kong SAR, Health and Medical Reasearch Fund
- 82171384/MOST | National Natural Science Foundation of China (NSFC)
- 20200730009/Guangzhou Key Projects of Brain Science and Brain-Like Intelligence Technology
- 2019SHIBS0001/Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions
- no/Nan Fung Life Sciences (NFLS)
- 2021A1515010856/GDSTC | Natural Science Foundation of Guangdong Province ()
- 15304020 R5013-19 R4005-18 and C4002-20WF/Hong Kong Research Grant Council
- 24117220/Hong Kong Research Grant Council
- 14117221/Hong Kong Research Grant Council
- AoE/M-604/16/Hong Kong Research Grant Council
- ITCPD/17-9/Innovation and Technology Commission (ITC)
- 06173946/Hong Kong Center for Neurodegenerative Diseases InnoHK of Hong Kong SAR, Health and Medical Reasearch Fund
- 20200730009/Guangzhou Key Projects of Brain Science and Brain-Like Intelligence Technology
- 2019SHIBS0001/Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

